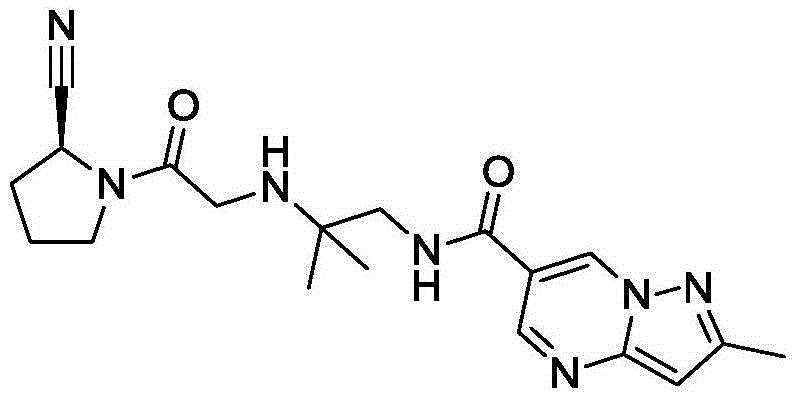
Preparation method of Anagliptin intermediate
A technology of intermediates and compounds, which is applied in the field of drug synthesis, can solve the problems that pyrimidine-6-carboxamide is not easy to obtain, and achieve the effects of no environmental pollution, simple operation, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
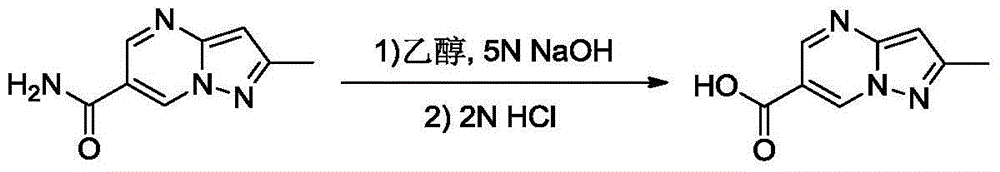
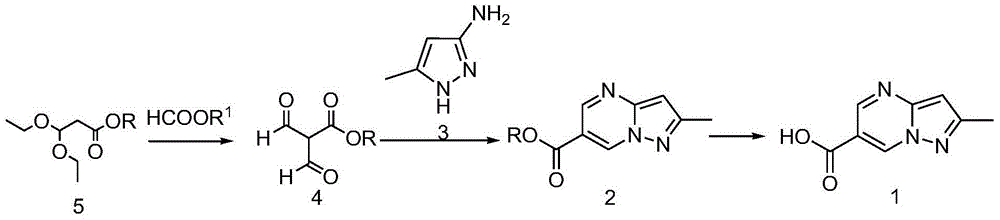
[0033] The preparation method of alalogliptin intermediate 1 comprises the following steps:
[0034]
[0035] In the first step, in the ether solution containing compound 5, a strong base was slowly added under ice bath conditions, then ethyl formate was added and stirred overnight at room temperature to obtain compound 4;
[0036] In the second step, compound 4 and compound 3 were added into the acidic solution, and stirred at 120° C. for 3 h to obtain compound 2;
[0037] The third step is to concentrate the above reaction solution, add ethanol and alkaline aqueous solution, heat to 70° C., and stir for 1 h to obtain compound 1.
[0038] In the first step, the volume molar ratio of ether to compound 5 is 2L / mol-3L / mol, and the strong base is 1.8-2 times the molar amount of compound 5. The temperature of the nucleophilic substitution reaction is -10°C-40°C, more preferably -5°C-15°C.
[0039] In the second step, the volume molar ratio of the acidic solution to the compou...
Embodiment 1
[0042] The synthesis of embodiment 1.2-formyl-3-oxopropionic acid ethyl ester
[0043]
[0044] Weigh 3,3-diethoxy ethyl propionate (10g, 52.57mmol), add diethyl ether (300ml), slowly add sodium hydride (4.2g, 105mmol) under ice-bath stirring conditions, then weigh ethyl formate Ester (39 g, 526 mmol) was added to the above reaction solution and stirred at room temperature overnight. After TLC (petroleum ether: ethyl acetate = 3:1) monitors the end of the reaction, transfer the reaction solution into a separatory funnel, add water (200ml), separate the water layer, adjust the pH of the water layer to 1 with hydrochloric acid, and then transfer to the separatory funnel. In a liquid funnel, extract with ether, wash the organic layer with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate to obtain an orange-red oily liquid. The obtained crude product can be directly put into the next reaction without purification.
Embodiment 2
[0045] The synthesis of embodiment 2.2-methyl-pyrazolo [1,5-a] pyrimidine-6-carboxylic acid ethyl ester
[0046]
[0047] Weigh 2-formyl-3-oxopropionic acid ethyl ester (3.2g, 32.95mmol) and 3-amino-5-methyl-1H-pyrazole (compound 4, 4.99g, 34.60mmol) in a three-necked flask In, acetic acid (40ml) was added, heated to 120°C and stirred for 3h. After the reaction was monitored by TLC (petroleum ether: ethyl acetate = 1:1), the heating was stopped, and the reaction solution was concentrated under reduced pressure to obtain the title crude product; the crude product could be directly put into the next reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com