Electrophosphorescent discoloration neutral iridium complex and preparation method thereof
An iridium complex, electrophosphorescence technology, applied in organic compounds/hydrides/coordination complex catalysts, color-changing fluorescent materials, compounds containing elements of Group 8/9/10/18 of the periodic table, etc. Problems such as complex chemical reactions, to achieve the effect of simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
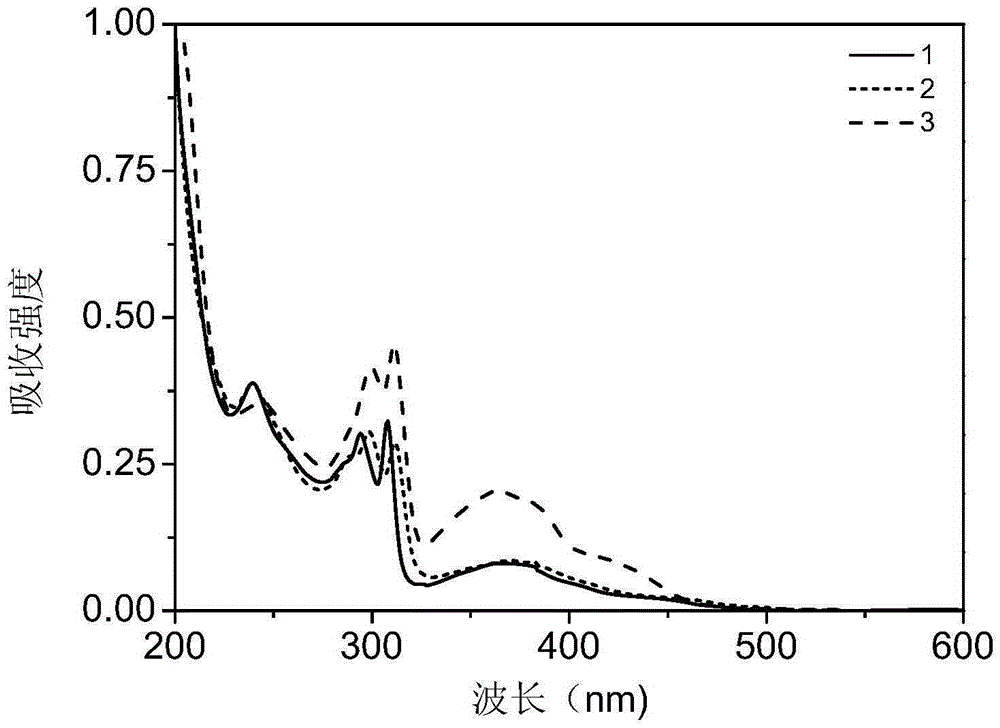
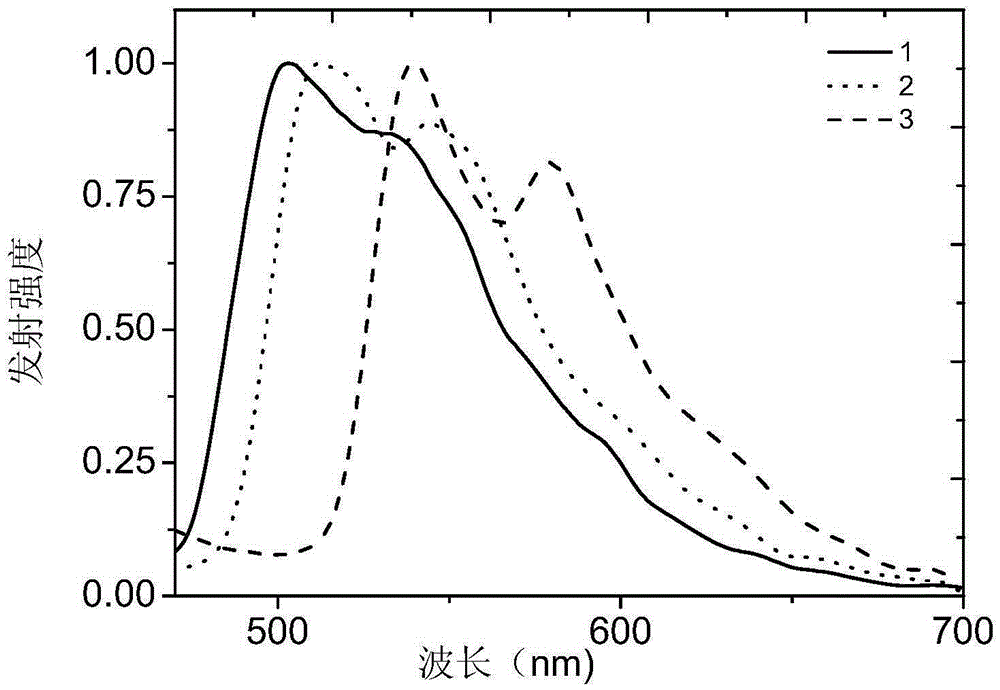
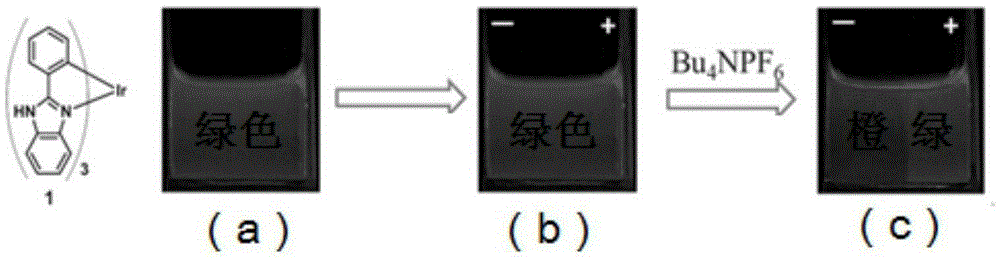
[0030] Embodiment 1: the preparation of complex 1
[0031]
[0032] Weigh 176.5mg (0.5mmol) of IrCl 3 ·3H 2 O and 194.2 mg (1.0 mmol) of 2-phenylbenzimidazole were added to a double-necked flask, vacuumed in the double-row tube-filled with nitrogen-vacuumized, and circulated three times in this way, and then under nitrogen protection, use a syringe After injecting 6mL of 2-ethoxyethanol and 2mL of water (3:1, v / v) respectively, the reaction mixture was heated to 110°C, and after stirring for 17-28 hours, the reaction mixture was cooled to room temperature and poured into the reaction solution After adding a large amount of water, the precipitate was filtered. The product was dried to give the iridium dichloro bridge compound as a yellow solid. Weigh the iridium dichloro bridge compound of 245.3mg (0.2mmol) and the potassium carbonate of 55.2mg (0.4mmol) and join in the double-necked flask, install the condenser tube, seal, vacuumize-fill nitrogen three times, weigh 40.6 ...
Embodiment 2
[0037] Embodiment 2: the preparation of complex 2
[0038]
[0039] Weigh 176.5mg (0.5mmol) of IrCl 3 ·3H 2 The 2-(4-trifluoromethylphenyl) benzimidazole of 0 and 262.3mg (1.0mmol) is added in the double-necked flask, vacuumizes-inflates nitrogen-vacuumizes in the double row tube, circulates like this three times, Then under the protection of nitrogen, after injecting 6mL of 2-ethoxyethanol and 2mL of water (3:1, v / v) respectively with a syringe, the reaction mixture was heated to 110 ° C, and after stirring for 17-28 hours, the reaction The mixture was cooled to room temperature, a large amount of water was added to the reaction liquid, and a precipitate was obtained by filtration. The product was dried to give the iridium dichloro bridge compound as a yellow solid. Weigh 300.4mg (0.2mmol) of iridium dichloride bridge compound and 55.2mg (0.4mmol) of potassium carbonate and join in the double-necked flask, install a condenser tube, seal, vacuum-fill nitrogen three times...
Embodiment 3
[0044] Embodiment 3: the preparation of complex 3:
[0045]
[0046] Weigh 176.5mg (0.5mmol) of IrCl 3 ·3H 2 O and 200.1mg (1.0mmol) of 2-(2-thienyl)benzimidazole were added to the double-necked flask, vacuumed in the double-row tube-filled with nitrogen-vacuumized, and circulated three times in this way, and then protected under nitrogen After injecting 6mL of 2-ethoxyethanol and 2mL of water (3:1, v / v) with a syringe, the reaction mixture was heated to 110°C, and after stirring for 17-28 hours, the reaction mixture was cooled to room temperature , After adding a large amount of water to the reaction liquid, the precipitate was obtained by filtration. The product was dried to give the iridium dichloro bridge compound as a yellow solid. Weigh the iridium dichloro bridge compound of 250.7mg (0.2mmol) and the potassium carbonate of 55.2mg (0.4mmol) and join in the double-necked flask, install the condenser tube, seal, vacuumize-fill nitrogen three times, weigh 40.6 Pour 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com