A kind of bimetallic catalyst for catalytic oxidation VOCs and its preparation method and application
A bimetallic catalyst and catalytic oxidation technology, which is applied in the field of resources and the environment, can solve the problems of transition metal oxide catalysis promotion and development obstacles, high reaction temperature, high energy consumption, etc., and achieve low cost, high catalytic activity and stable conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] This example prepares a bimetallic catalyst for catalytic oxidation of VOCs, including a carrier, an active agent and a cocatalyst. The carrier is titanium dioxide, the activator is ruthenium, and the cocatalyst is tricobalt tetroxide. The percentage of the total mass is 1.0 wt%, and the atomic molar ratio of the ruthenium element in the active agent to the cobalt element in the cocatalyst is 1:5.
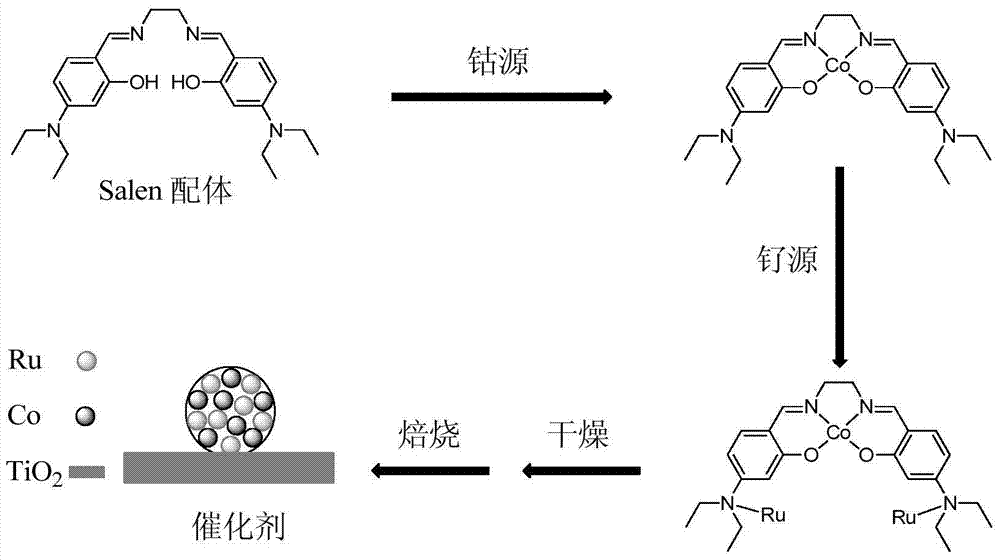
[0061] The preparation method of described catalyst is as follows (flow process is as follows figure 1 shown):
[0062] (1) Dissolve Salen ligand and cobalt acetate in ethanol (the molar ratio of Salen ligand to cobalt acetate is 1:1), and heat and stir at 50°C for 5 hours to obtain the coordinated single metal Organic compound ethanol solution (wherein the concentration of ethanol solution is 0.5g / mL).
[0063] (2) Ruthenium nitrosyl nitrate is added to the ethanol solution of the monometallic organic compound obtained in step (1) (wherein the mol ratio of ruthenium nitro...
Embodiment 2
[0067] In addition to the percentage of ruthenium element accounting for the total mass of the catalyst in the active agent is 0.2wt%, the atomic molar ratio of the ruthenium element in the activator and the cobalt element in the cocatalyst is 1:1, and in the step (1), the Salen ligand and cobalt acetate The mol ratio is 1:3, the concentration of the ethanol solution in the monometallic organic compound ethanol solution is 0.1g / mL, the ruthenium source described in step (2) is ruthenium trichloride, the mol ratio of ruthenium trichloride and cobalt acetate The concentration of titanium dioxide in the titanium dioxide slurry in step (3) is 1:1, except that the concentration of titanium dioxide in the slurry of titanium dioxide is 0.1 g / mL, the dosage of other materials and the preparation steps are the same as in Example 1.
[0068] The particle size of the bimetallic active particles of the obtained catalyst is 10nm, and when it catalyzes the simulated flue gas containing 1000p...
Embodiment 3
[0070] In addition to the percentage of ruthenium element accounting for the total mass of the catalyst in the active agent is 2wt%, the atomic molar ratio of the ruthenium element in the activator and the cobalt element in the cocatalyst is 1:10, the Salen ligand and cobalt acetate in the step (1) The mol ratio is 1:5, and the concentration of the ethanol solution in the monometallic organic compound ethanol solution is 1g / mL, and the mol ratio of ruthenium nitrosyl nitrate and cobalt acetate is 1:10 in the step (2), and titanium dioxide is 1:10 in the step (3). Except that the concentration of titanium dioxide in the slurry is 1g / mL, other material dosages and preparation steps are the same as in Example 1.
[0071]The particle size of the bimetallic active particles of the obtained catalyst is 10nm, and its complete oxidation temperature is 225°C, 210 °C and 215 °C, CO 2 Selectivity ≥ 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com