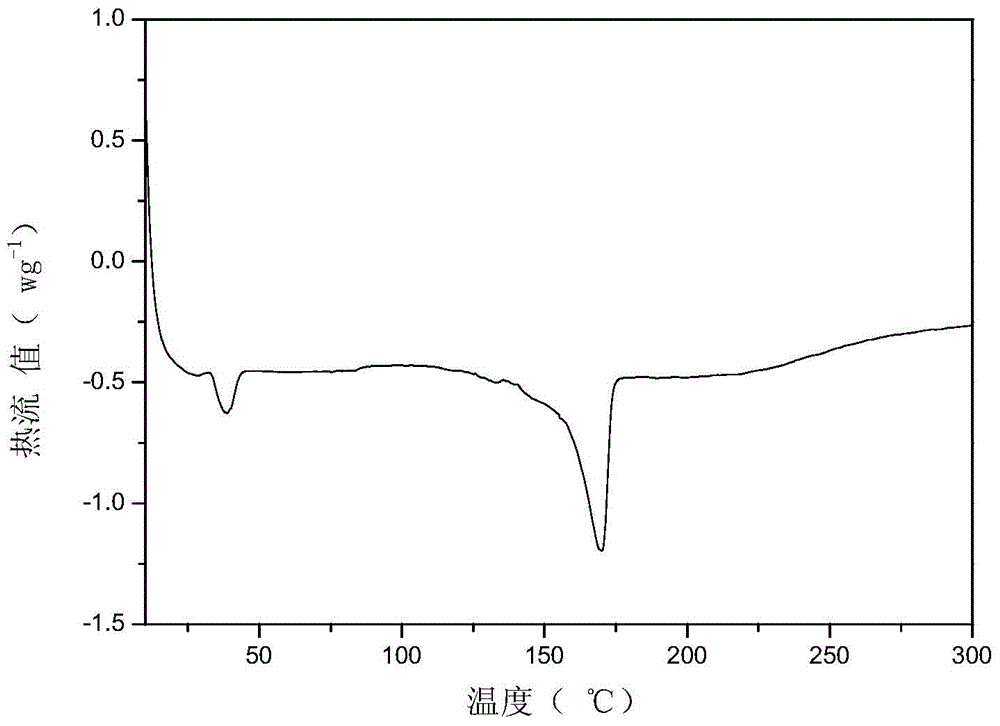
Aromatic ether nitrile-group resin monomer and synthesis method thereof
A synthesis method and resin-based technology, applied in chemical instruments and methods, carboxylic acid nitrile preparation, organic compound preparation, etc., can solve the problems of high post-curing temperature, long curing time, and low relative content of nitrile groups, etc. Achieve the effects of short post-curing time, simple and effective synthesis method, and simple and easy-to-control synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
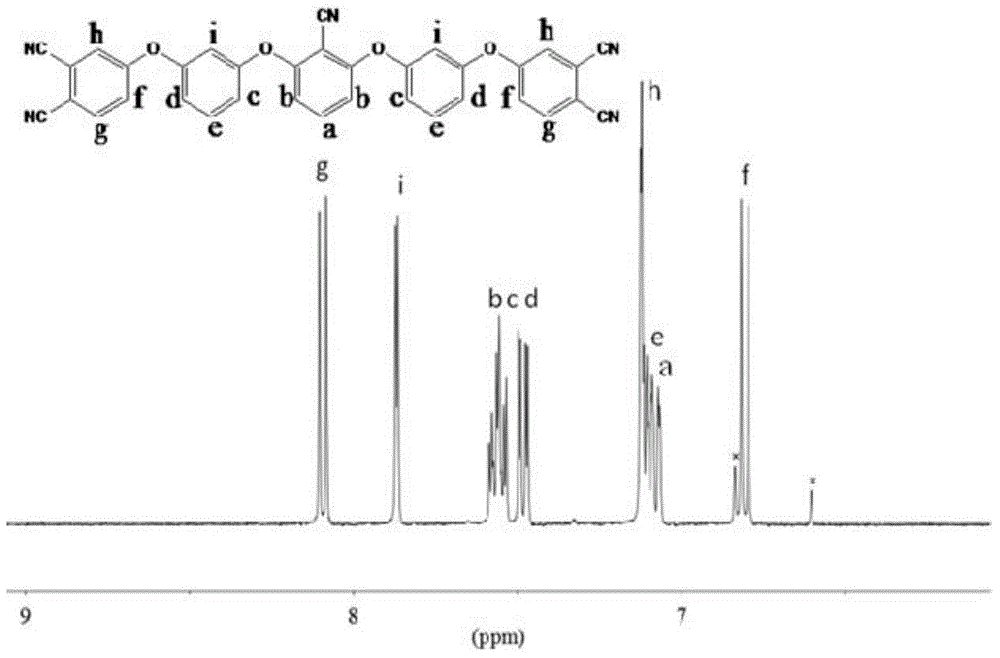
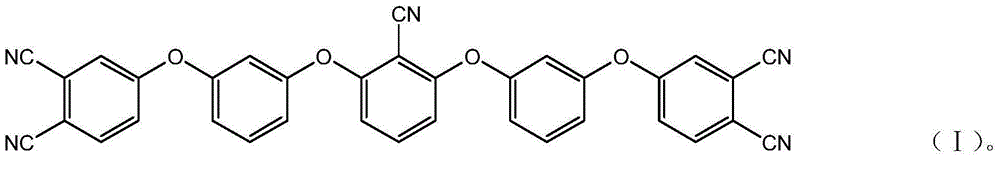
[0035] A kind of aryl ether nitrile base resin monomer of the present invention, molecular structural formula is following formula (I):
[0036]
[0037] A kind of synthetic method of the aromatic ether nitrile resin monomer of above-mentioned present embodiment, comprises the following steps:
[0038] (1) Preparation of 2,6-bis(3-oxyphenoxy)benzonitrile salt
[0039] (1.1) Add 0.006mol resorcinol, 0.012mol anhydrous potassium carbonate, 20ml N-methylpyrrolidone NMP (solvent), 20ml toluene (water-carrying agent), into a dry reactor (such as a three-neck round bottom flask) , and install an upper water separator and a reflux condenser, vacuumize and replace nitrogen, heat the oil bath to 140 ° C, and keep stirring and reflux for 3 hours. During this period, the mixed solution of water agent and water is continuously divided.
[0040] (1.2) When the mixture of water-carrying agent and water no longer appears in the reflux condenser, raise the temperature to 170°C to evapora...
Embodiment 2
[0048] A kind of aryl ether nitrile resin monomer of the present invention is identical with embodiment 1.
[0049] A kind of synthetic method of the aryl ether nitrile resin monomer of the present embodiment, step is basically the same as embodiment 1, difference only is: in step (1.1), the solvent that adopts is dimethyl sulfoxide DMSO; In step (1.2) The temperature for steaming and removing the water-carrying agent is 160° C.; the reflux temperature in step (1.3) is 180° C., and the reflux stirring time is 12 hours.
Embodiment 3
[0051] A kind of aryl ether nitrile resin monomer of the present invention is identical with embodiment 1.
[0052] A method for synthesizing the aryl ether nitrile resin monomer of this embodiment, the steps are basically the same as in Example 1, the only difference being that in step (1.1), the oil bath heating temperature is 150°C and kept for 1 hour.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com