Treatment of pediatric growth hormone deficiency with human growth hormone analogues
A growth hormone deficiency, human growth hormone technology, applied in the field of application of patients with D, can solve problems such as joint pain, nausea, vomiting and injection reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0296] Example 1A - Single Dose Results
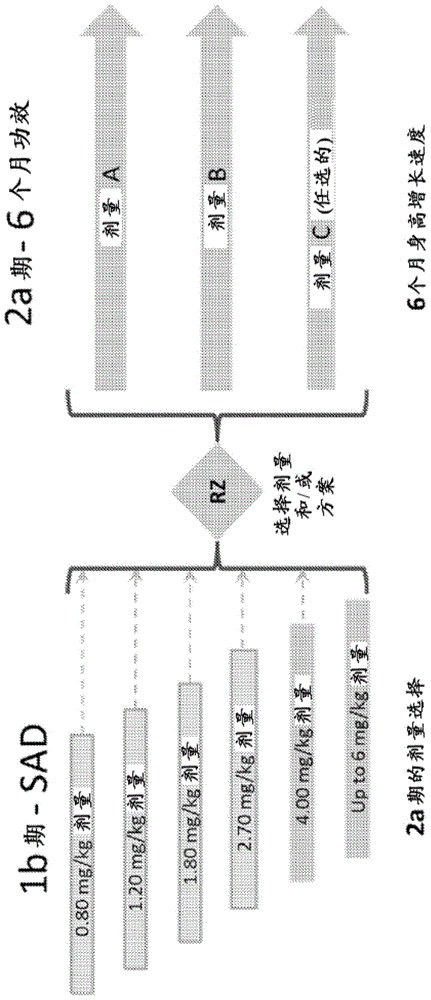
[0297] Studies have been conducted on single-dose human growth hormone analogues ( figure 1 Safety, pharmacokinetics (PK) and pharmacokinetics of the human growth hormone-XTEN (hGH-XTEN) fusion protein shown as SEQ ID NO: 1 when subcutaneously (SC) administered in pediatric patients with growth hormone deficiency. Phase 1b / 2a trial of efficacy (PD). Based on the safety profile in adults with GHD (Yuen, K.C. et al., The Journal of Clinical Endocrinology and Metabolism 98, 2595-2603 (2013)) and the possibility of achieving monthly dosing, the phase 1b / 2a study in children with GHD determined (i) safety, Tolerance, PK, and IGF-I response in children with GHD to a single dose of hGH-XTEN fusion protein (Phase 1b); and (ii) 6-month height using a fusion protein dosing regimen that normalizes IGF-I Growth rate (Phase 2a).
[0298] The study was designed to enroll 72 treatment-naive prepubertal children. Key inclusion criteria were prep...
Embodiment 1B
[0316] Example 1B - Single Dose Results
[0317] Currently approved growth hormone drugs require daily injections and thus present considerable challenges to patients with GHD. In contrast, human growth hormone analogs (( figure 1 ) shown as a human growth hormone-XTEN (hGH-XTEN) fusion protein of SEQ ID NO: 1) was developed to provide up to once-monthly dosing, helping to increase the ability of patients to comply with their regimen and improve their overall treatment outcome .
[0318] Data were collected from a phase 1b / 2a study of a novel long-acting human growth hormone (hGH-XTEN fusion protein) in prepubertal children with growth hormone deficiency (GHD). The purpose of the phase 1b study was to evaluate the single-dose safety, tolerability of hGH-XTEN fusion protein in pediatric patients with GHD; and to determine the PK (hGH-XTEN fusion protein concentration) and PD (IGF-I, IGFBP- 3) Features.
[0319] The clinical trial enrolled up to 72 treatment-first prepuber...
Embodiment 2
[0332] Example 2 - Repeat Dosing Results
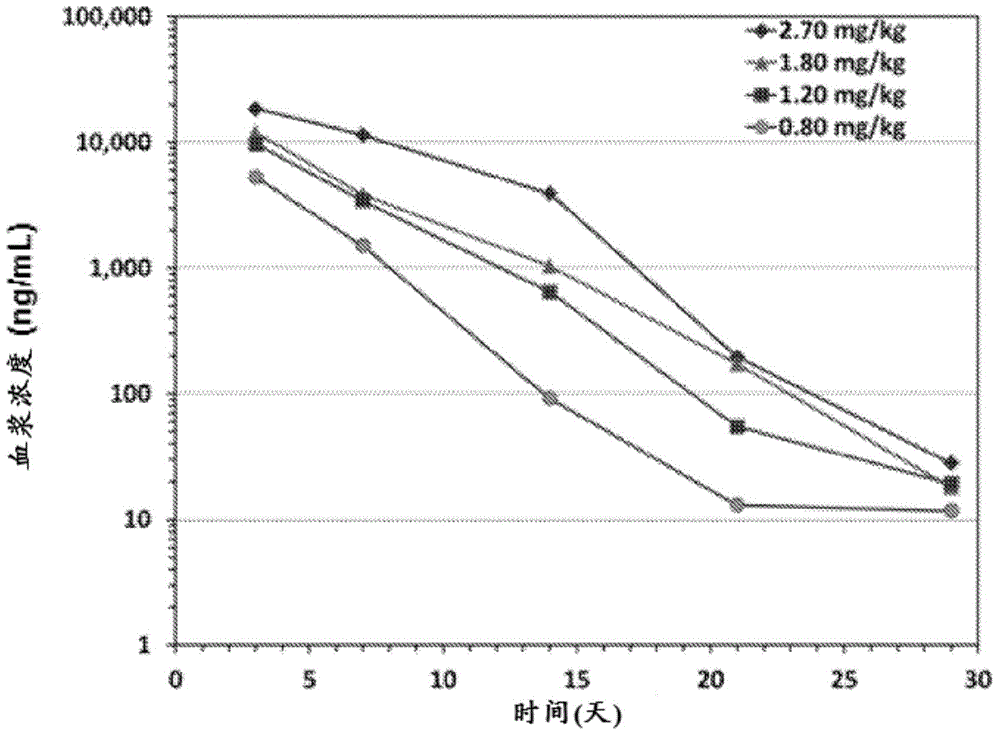
[0333] VRS-317 is an amino acid sequence (XTEN) ( figure 1 A novel fusion protein (M.W.119kDa) consisting of SEQ ID NO: 1). In a phase 1 study in adults and children with GHD, VRS-317 concentrations, IGF-I and IGFBP-3 responses were dose proportional, and drug concentrations and increases in IGF-I and IGFBP-3 were sustained after a single subcutaneous injection Present for 30 days. Single-dose VRS-317 administration was safe and well tolerated, with minor injection site discomfort; no new safety signals compared to those already presented with daily rhGH products.
[0334] A repeat dosing study was conducted to determine safety, tolerability, height growth velocity, IGF-I and IGFBP-3 responses after 6 months of VRS-317 treatment. The primary endpoint was mean 6-month height growth velocity. The subjects were all prepubescent and receiving rhGH treatment for the first time. GHD is diagnosed by the following: short stature (HT-SD...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com