Dimethyl phthalate artificial immunogen and preparation and application thereof
A technology of dimethyl phthalate and artificial immunogen, which is applied in the field of dimethyl phthalate artificial immunogen DMP-BSA and its preparation, to achieve the advantages of strong antigen practicability, simple and feasible preparation technology, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, the synthesis of dimethyl phthalate hapten
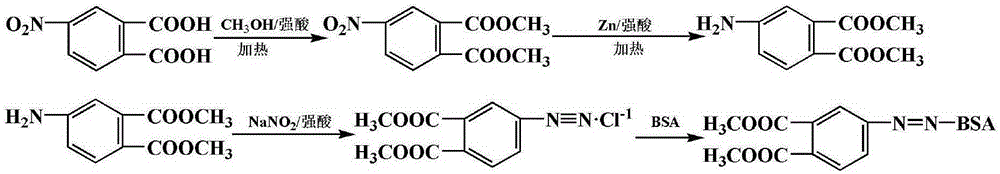
[0054] The synthetic route diagram of dimethyl phthalate hapten is as follows figure 1 Shown:
[0055] (1) Synthesis of dimethyl phthalate hapten intermediate
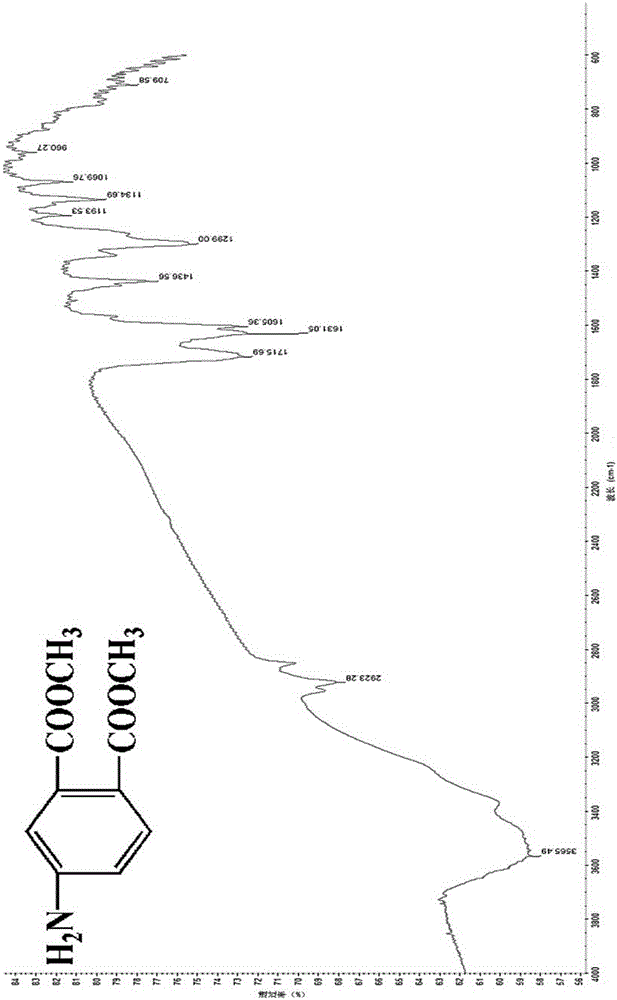
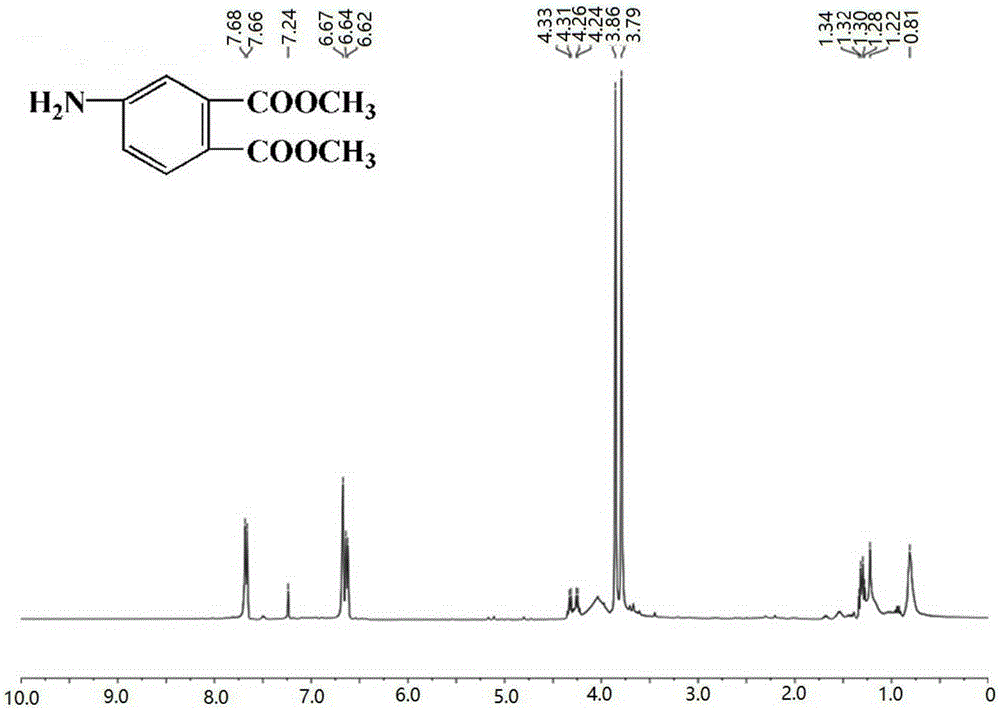
[0056] Weigh 10g (0.0474mol) of 4-nitrophthalic acid into a round bottom flask, then slowly add 11.5mL (0.2850mol) of methanol, and add 1.65mL (0.0304mol) of concentrated H 2 SO 4 Catalyze, gradually heat up to 90°C and heat under reflux for 5 hours until the raw material point disappears in thin plate chromatography (TLC) detection (chromogenic agent: dissolve a small amount of potassium permanganate in acetone solvent; developing agent, n-hexane: ethyl acetate =5:1); after the reaction, the reaction solution was transferred to a rotary evaporator to distill off unreacted methanol and generated water, then the liquid was poured into ice water while hot, and the solid was precipitated; then the solid crude product was washed with 10% Na 2 CO 3 Th...
Embodiment 2
[0059] Embodiment 2, the synthesis of dimethyl phthalate hapten
[0060] The synthetic route diagram of dimethyl phthalate hapten is as follows figure 1 Shown:
[0061] (1) Synthesis of dimethyl phthalate hapten intermediate
[0062] Weigh 10g (0.0474mol) of 4-nitrophthalic acid into a round bottom flask, then slowly add 9.6mL (0.2370mol) of methanol, and add 1.67mL (0.0308mol) of concentrated H 2 SO 4 Catalyze, gradually warming up to 80°C and heating under reflux for 6 hours until the point of the raw material detected by thin plate chromatography (TLC) disappears (chromogenic agent: a small amount of potassium permanganate dissolved in acetone solvent; developing agent, n-hexane: ethyl acetate =5:1); after the reaction, the reaction solution was transferred to a rotary evaporator to distill off unreacted methanol and generated water, then the liquid was poured into ice water while hot, and the solid was precipitated; then the solid crude product was washed with 10% Na...
Embodiment 3
[0065] Embodiment 3, the synthesis of dimethyl phthalate hapten
[0066] The synthetic route diagram of dimethyl phthalate hapten is as follows figure 1 Shown:
[0067] (1) Synthesis of dimethyl phthalate hapten intermediate
[0068] Weigh 10g (0.0474mol) of 4-nitrophthalic acid into a round bottom flask, then slowly add 12.4mL (0.3081mol) of methanol, and add 1.80mL (0.0332mol) of concentrated H 2 SO 4Catalyze, gradually heat up to 100°C and heat under reflux for 5 hours, until the raw material point detected by thin plate chromatography (TLC) disappears (chromogenic agent: dissolve a small amount of potassium permanganate in acetone solvent; developing agent, n-hexane: ethyl acetate =5:1); after the reaction, the reaction solution was transferred to a rotary evaporator to distill off unreacted methanol and generated water, then the liquid was poured into ice water while hot, and the solid was precipitated; then the solid crude product was washed with 10% Na 2 CO 3 Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com