GnRH analogue-cytotoxic molecule conjugate and preparation method and application thereof
A compound and small molecule technology, applied in the fields of chemistry and medicine, can solve problems such as toxic side effects and low stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Example 1: Synthesis of Peptide Sequences
[0157] Using 0.5g MBHA resin (0.54mmol) as a solid phase carrier, DIC / HOBt as a condensing agent, according to the amino acid sequence of the compound, all amino acids and modification units are connected sequentially according to the Boc and Fmoc cross-protection strategies, and the above peptide resin is put into HF cutting Add 1.0mL of anisole and 0.5mL of ethanedithiol to the reactor of the instrument. After installation, the system of the HF cutting instrument is evacuated, and the reactor is cooled with liquid nitrogen, and about 10mL of liquid HF is transferred. Reaction 1h. Use an oil pump to pump out HF, remove the reactor, add frozen anhydrous ether to precipitate a solid, and then transfer the suspension to a funnel with a sand core. Wash three times with a small amount of cooled anhydrous ether, and then rinse with 10% acetic acid aqueous solution until the resins no longer adhere to each other, collect the washin...
Embodiment 2
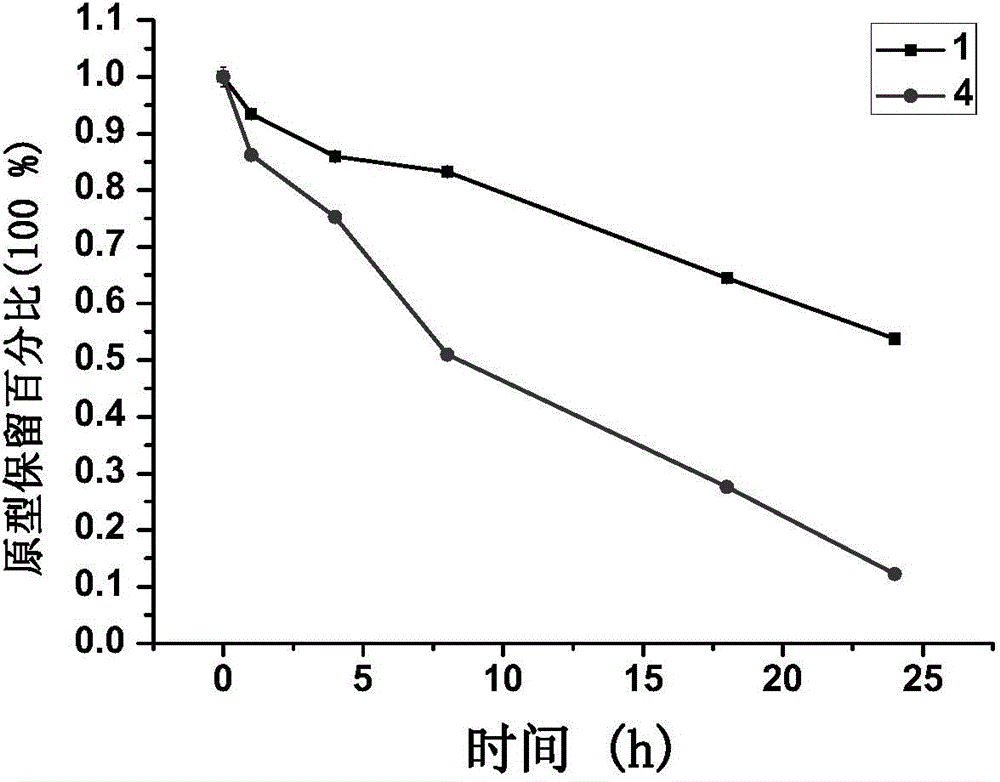
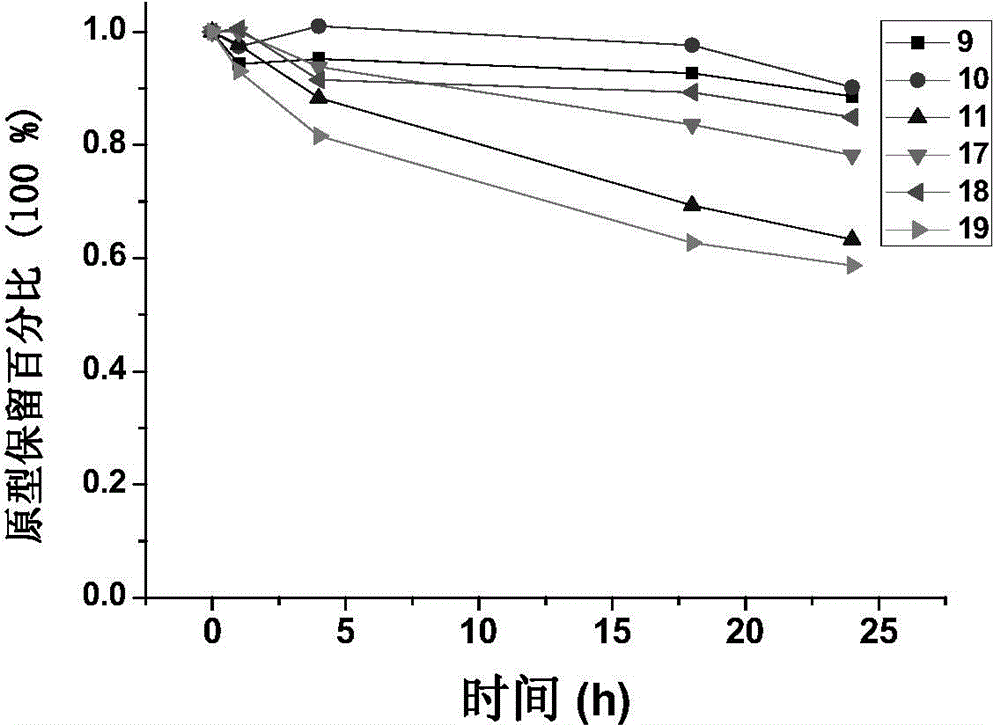
[0160] Example 2: Synthesis of GnRH analogue-paclitaxel conjugates linked by thioether bonds
[0161] Weighing equimolar amounts of the peptide sequence and paclitaxel derivative A in 50% acetonitrile / water solution, reacting with stirring at room temperature, after the reaction was complete, purified by medium-pressure preparative chromatography to obtain a pure product.
[0162] Conjugates were synthesized in this way: 1, 9, 10, 11, 17, 18, 19.
Embodiment 3
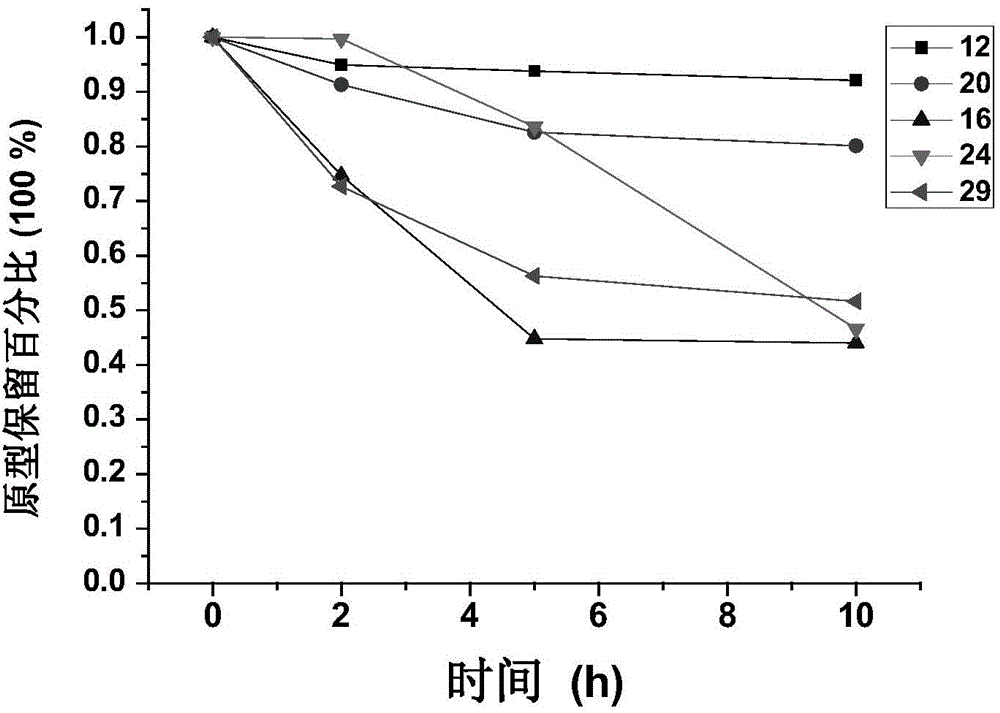
[0163] Example 3: Synthesis of Disulfide-Linked GnRH Analogue-Paclitaxel Conjugates
[0164] Weigh the equimolar amount of peptide sequence and paclitaxel derivative B in DMSO, react with stirring at room temperature, after the reaction is complete, purify by medium pressure preparative chromatography to obtain pure product.
[0165] Conjugates were synthesized in this way: 4, 12, 16, 20, 24, 29.
[0166] The mass spectrum characterization data of Example 2 and Example 3 are shown in Table 2.
[0167] Table 2 GnRH analogs - Maldi-Tof-MS Characterization of Paclitaxel Conjugates
[0168]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com