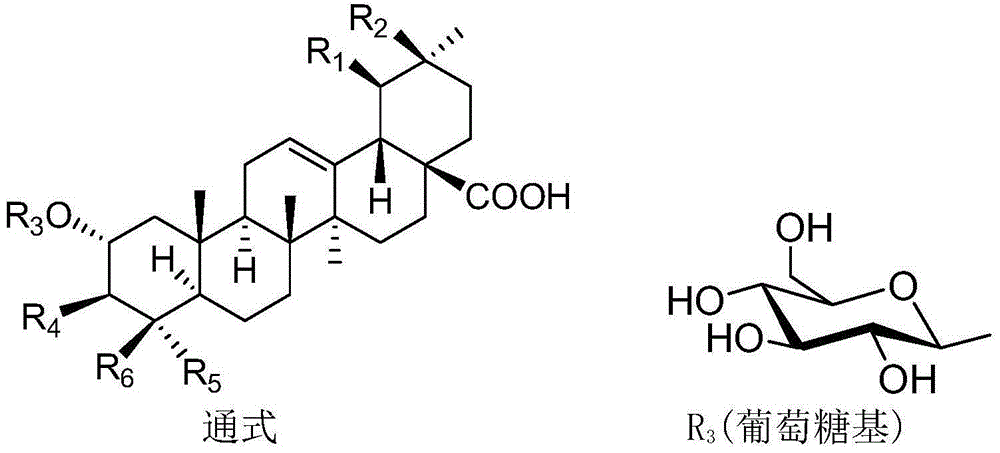
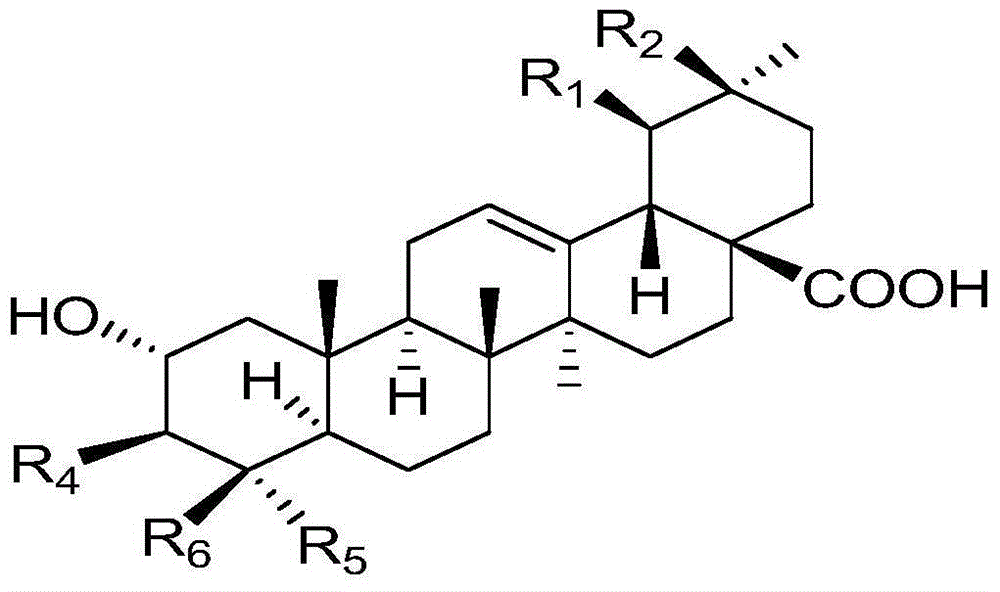
Polyglycosidated pentacyclic triterpene-28-acid, and preparation method and application thereof
A pentacyclic triterpene and polysaccharide technology, which is applied in the field of medicine and its preparation and application, can solve the problems of restricted application, poor water solubility, and low bioavailability, and achieve the effects of small toxic and side effects, enhanced water solubility, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of synthesis of 2α, 3β-2-(O-β-D-glucopyranoside) oleanane-28-acid, the specific steps are as follows:
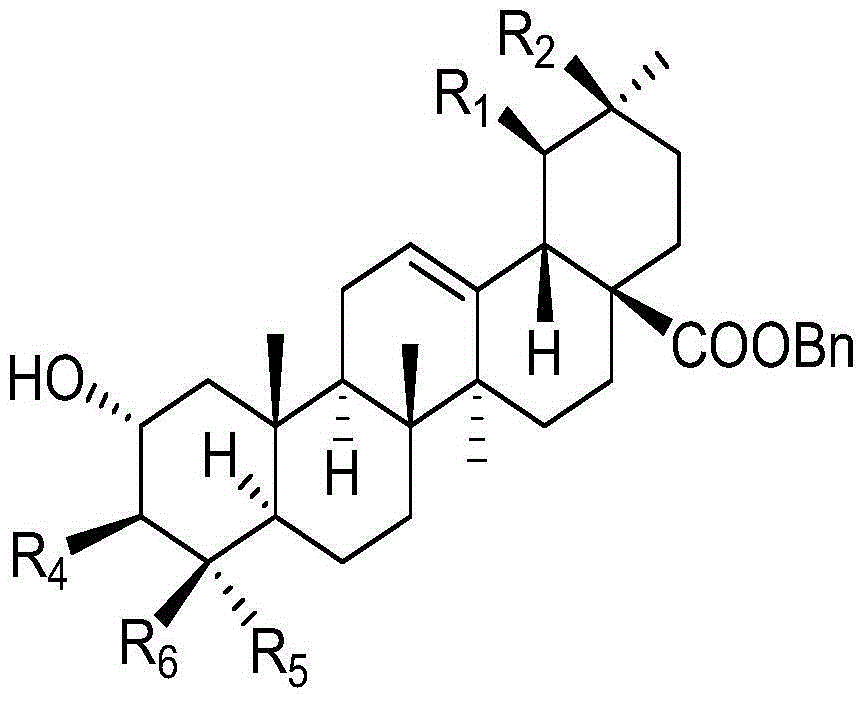
[0039] (1) Synthesis of 2α, 3β-dihydroxy-oleanane-28-acid benzyl ester:
[0040]
[0041]2a-Hydroxyoleanolic acid (10g, 21.2mmol) and potassium carbonate (6g, 43.6mmol) were dissolved in 50mL of DMF, and 4.2mL of BnBr was added, and the reaction was stirred for 24h at a reaction temperature of 50°C. Add distilled water, wait for the solid to precipitate, filter, and dry to obtain 11.0 g of the target product, the yield is 92.0%, and the melting point is 155-157 ° C;
[0042] (2) Synthesis of 2α,3β-di-O-(2,3,4,6-4-O-benzoyl-β-D-glucopyranoside)-oleanane-28-oic acid benzyl ester :
[0043]
[0044] The product of step (1) (1 g, 1.8 mmol) and trichloroacetimidoglucose ester (2.94 g, 3.95 mmol) were dissolved in dichloromethane, and 5 drops of TMSOTf were added, and stirred at room temperature for 24 h. After filtration and concentration under reduced pres...
Embodiment 2
[0053] Synthesis of 2α,3β-di-O-(β-D-galactopyranoside)-(1→4)-(β-D-glucopyranoside)-ursane-28-acid, specific synthesis steps With embodiment 1.
[0054] Melting point: 291-293°C.
[0055] Spectral data: 1 HNMR (400MHz, DMSO-d 6 ):δ5.10(s,1H),4.47(d,J=7.5Hz,1H),4.36(d,J=7.0Hz,1H),4.18-4.15(m,2H),3.90-3.88(m, 1H),3.76-3.74(m,2H),3.62-3.59(m,4H),3.58-3.54(m,4H),3.53-3.50(m,4H),3.49-3.42(m,8H),3.33- 3.28(m,9H),3.27-3.21(m,6H),3.12-3.06(m,2H),2.13-2.12(m,2H),1.91-1.83(m,4H),1.73(s,2H), 1.55-1.39(m,8H),1.26-1.21(m,5H),1.05(s,3H),1.01(s,3H),0.88(s,6H),0.79(s,3H),0.76(s, 3H),0.74(s,3H). 13 CNMR (100MHz, DMSO-d 6 ): δ176.2, 138.8, 124.0, 104.1, 103.3, 98.3, 87.2, 81.4, 80.9, 79.2, 75.6, 75.2, 75.0, 74.8, 74.7, 74.1, 73.3, 72.8, 71.5, 70.7, 70.6, 68.2, 60.9, 60 MS( HRMS):m / z[M+Na] + calcdforC 54 h 88 o 24 Na: 1143.5558; found: .1143.5508.
Embodiment 3
[0057] The polyglycosidated pentacyclic triterpene-28-acid synthesized in Examples 1 and 2 was tested for α-glucosidase inhibitory activity and its antidiabetic activity was evaluated.
[0058] α-Glucosidase inhibition model.
[0059] The total volume of the reaction system is 250 μL, the blank reaction system: 10 μL 1U / mL α-glucosidase, 220 μL 67 mmol / LKH 2 PO 4 -K 2 HPO 4 buffer and 20 μL of 5.8 mmol / L p-nitrophenyl-α-D-glucopyranoside (PNPG) solution.
[0060] Prepare a series of concentration gradients of 2α,3β-bis-(O-β-D-glucopyranoside)-oleanane-28-acid, add to the solution containing 10 μL 1U / mL α-glucosidase and a certain amount of buffer solution, incubated at 37°C and pH=6.8 for 10 minutes, and finally 20 μL of PNPG solution was quickly added to the reaction system as a substrate. 4 parallel experiments.
[0061] Inhibition rate = [(A-(A 1 -A 2 )) / A]×100%, A is the absorbance value measured by the blank reaction system, A 1 Absorbance value measured for the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com