Preparation method of sofosbuvir
A technology of compound and alkyl, applied in the field of drug synthesis, can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation of embodiment 1 Sofosbuvir
[0064]
[0065] Step (a):
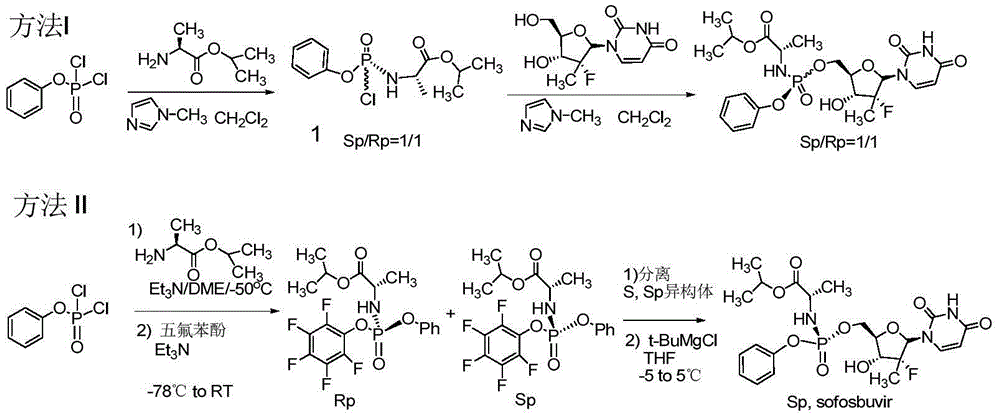
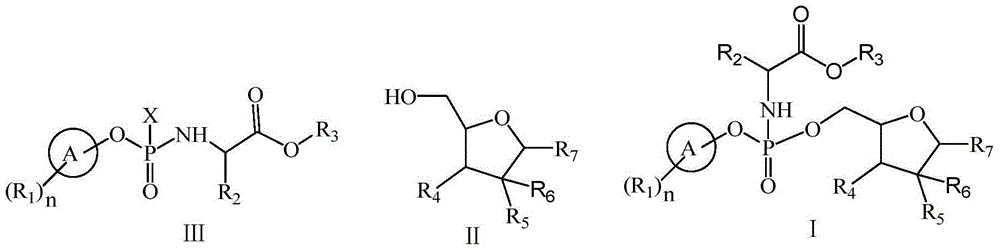
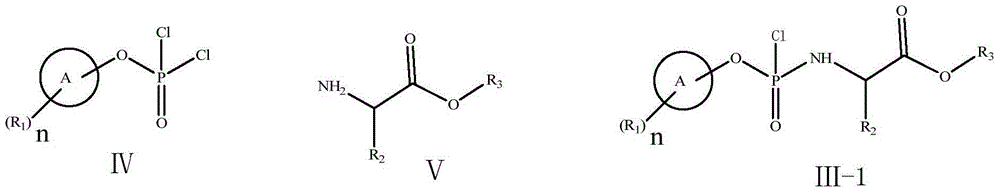
[0066] At 0°C, phenyl dichlorophosphate (6.0g, 28.4mmol) was dissolved in anhydrous dichloromethane (30ml), and alanine isopropyl hydrochloride (4.8g, 28.4mmol) was added under stirring, and the mixture was After stirring and cooling to -55°C, a mixed solution of triethylamine (6.5g, 64mmol) and dichloromethane (30ml) was slowly added dropwise, keeping the temperature at -55°C during the period. Stir at -5°C for 2 hours, and monitor the completion of the reaction by TLC. Triethylamine hydrochloride was removed by filtration, and the solvent was evaporated from the filtrate under reduced pressure to obtain compound 3-1 as a colorless oil (Sp / Rp=1 / 1).
[0067] 31 PNMR (CDCl 3 ,300Hz,H 3 PO 4 internal standard): δ8.25&7.94(1:1);
[0068] 1 HNMR (CDCl 3 ,300MHz):δ7.39-7.34(m,2H),7.27-7.18(m,3H),5.10-5.02(m,1H),4.51(br,1H),4.11(m,1H),1.49(d ,3H),1.29-1.24(m,6H);
[0069] 13 CNMR (CDCl 3 ...
Embodiment 2
[0075] The preparation of compound shown in embodiment 2 formula 3-2
[0076]
[0077] (1) The nucleophile is NaSCN, and the phase transfer catalyst is TBAB
[0078] The compound shown in formula 3-1 (the product of step (a) of Example 1) was dissolved in dichloromethane (20ml), TBAB (2.8mmol) was added, and a solution of NaSCN (35mmol) in water (2.0ml) was added dropwise Added to the above reaction solution. After dropping, stirring was continued for 60 minutes, and the solid was removed by filtration. After washing the filtrate with water, add MgSO 4 Let dry for 24 hours. After filtration, the filtrate was evaporated to remove the solvent under reduced pressure to obtain the compound shown in formula 3-2 (wherein X=SCN).
[0079] 1 HNMR (CDCl 3 ,500Hz):δ7.32-7.13(m,3H),7.08-7.02(m,2H),5.0-4.9(m,1H),3.92(m,1H),1.49(m,3H),1.23-1.17 (m,6H);
[0080] 31 PNMR (CDCl 3 ,300Hz,H 3 PO 4 as internal standard): δ-18.16 / -18.26.
[0081] (2) The nucleophile is NaSCN, and ...
Embodiment 3
[0093] The preparation of embodiment 3 Sofosbuvir
[0094]
[0095] (1) X is SCN
[0096] At 5°C, the compound represented by formula 2 (5.20 g, 20.0 mmol) was dissolved in anhydrous THF (30 ml). Tert-butylmagnesium chloride (1.0M solution in THF, 42ml, 42.0mmol) was added with stirring. The reaction temperature was raised to 25°C, and the mixture was stirred for 30 minutes. After adding lithium chloride (21.0 mmol), a mixed solution of the compound of formula 3-2 (about 28.4 mmol, prepared in Example 2) and THF (30 ml) was slowly added dropwise, while keeping the temperature at 5°C. After dropping, it was stirred for 15 hours. The reaction solution was quenched with 1N HCl aqueous solution (25 ml) (the ratio of Sp:Rp determined by HPLC was 6:1). After additional toluene (100ml) was added, the temperature rose to room temperature. Organic layer with 1N HCl, water, 5% Na 2 CO 3 and brine, dried over anhydrous magnesium sulfate, filtered, evaporated under reduced press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com