A kind of method for preparing epichlorohydrin
A technology of dichloropropanol and oxides, applied in the direction of organic chemistry, can solve the problems of high salt content industrial wastewater, many by-products, etc., and achieve the effect of high catalytic activity, less side reactions and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] This example illustrates the reaction process and results when the catalyst is 5CaO / 80NaZSM-5 (the mass of supported CaO is 5% of the mass of NaZSM-5, Si / Al=80).
[0019] (1) First prepare 500ml of NaNO with a concentration of 1mol / L 3 Solution, then take 3g of HZSM-5 (Si / Al=80) molecular sieve powder and dissolve in sufficient amount of the above solution, stir at room temperature for 2~3h, repeat 4 times, after suction filtration and drying, roast at 550°C 6h to get 80NaZSM-5 sample.
[0020] (2) Add 0.4211g of Ca(NO 3 ) 2 4H 2 O was dissolved in 2.5ml of distilled water corresponding to the saturated water absorption volume of 80NaZSM-5, and then 2g of 80NaZSM-5 powder was mixed in the above solution, after drying, it was calcined in air at 550°C for 7 hours to obtain 5CaO / 80NaZSM-5 catalyst powder.
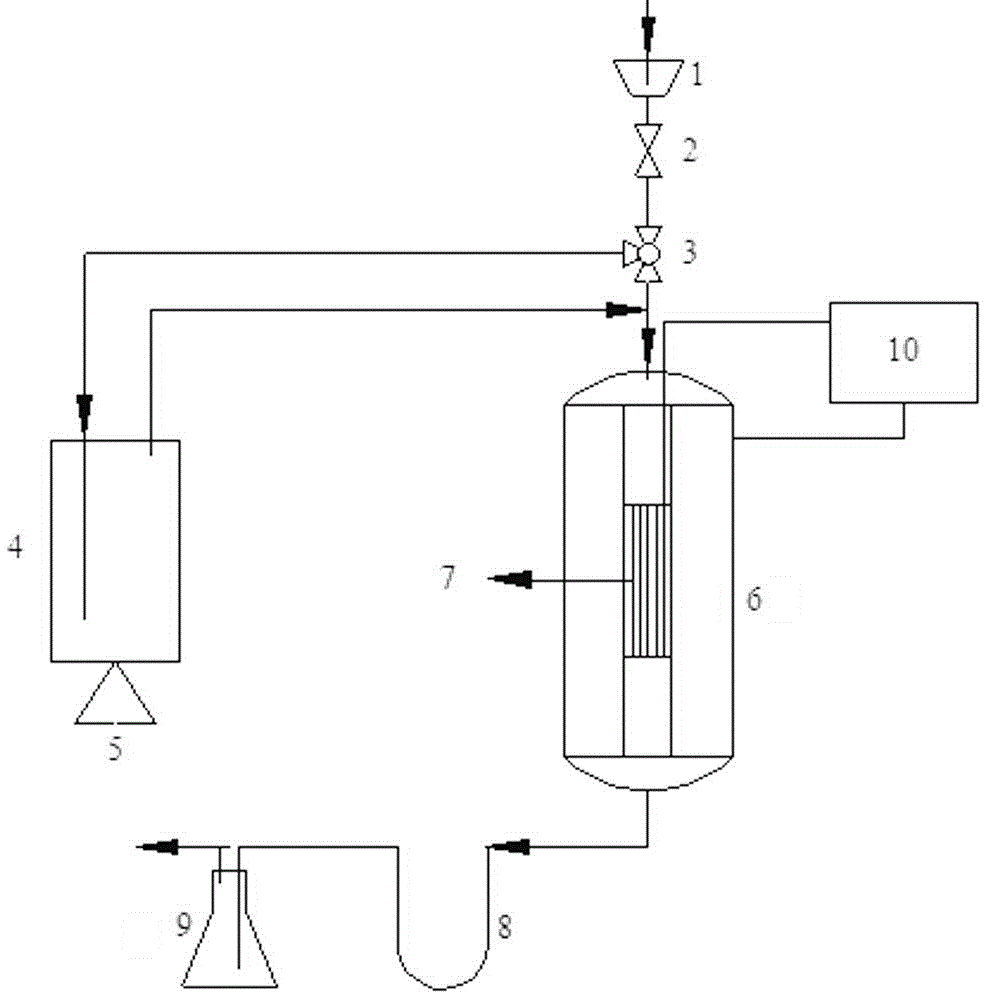
[0021] (3) Press the catalyst obtained above into tablets with a powder tablet press with a pressure of 6 MPa and a holding time of 1 min, and then grind and scree...
Embodiment 2
[0025] This example illustrates the reaction process and results when the catalyst is HY.
[0026] Press the HY molecular sieve powder into tablets, screen out 0.3g of HY molecular sieve particles with a diameter of 80-100 mesh, put the weighed HY molecular sieve particles into the reaction tube (Ф8 mm×1 mm×250 mm), and the temperature of the saturator is 60 °C, the carrier gas flow rate is 200ml / min, and other steps are the same as (4) in Example 1.
[0027] temperature(℃) Conversion rate / % Selective / % Yield / % 300 32.0 83.1 26.3 330 32.4 80.0 26.0 360 32.9 76.0 25.1 390 18.1 61.6 11.1
Embodiment 3
[0029] This example illustrates the reaction process and results when the catalyst is 10MgO / 25NaZSM-5 (the mass of supported MgO is 10% of the mass of NaZSM-5, Si / Al=25).
[0030] The Na exchange process of HZSM-5 (Si / Al=25) molecular sieve is the same as that of Example 1 (1), and 1.2722g of Mg(NO 3 ) 2 ·6H 2 O was dissolved in 2.5ml of distilled water corresponding to the saturated water absorption volume of 25NaZSM-5, and 2g of 25NaZSM-5 powder was mixed in the above solution, and after drying, it was calcined in air at 550°C for 7 hours to obtain 10MgO / 25NaZSM-5 catalyst powder. Other steps are the same as steps (3) (4) in Example 1. The result is as follows:
[0031] temperature(℃) Conversion rate / % Selective / % Yield / % 300 91.7 95.3 87.4 330 97.1 96.8 93.9 360 98.2 96.6 94.9 390 62.7 93.5 58.6
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com