Enzyme with function of catalyzing formaldehyde for synthesis of 1,3-dihydroxyacetone and preparation method of enzyme
A technology of condensation of dihydroxyacetone and formaldehyde, applied in the biological field, can solve the problems of incapable of industrialized production and many side reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
[0035] Example 1 BFD gene acquisition, vector construction
[0036] The source of BFD species is Pseudomonasputida, and its gene sequence is shown in SEQIDNO.2. Under the premise of not changing the amino acid sequence of BF, the codons of the above wild-type gene were replaced with the codons of Escherichia coli preference (high frequency use). After codon optimization, the gene sequence has Escherichia coli preferred codons, and its gene sequence is shown in SEQ ID NO.3.
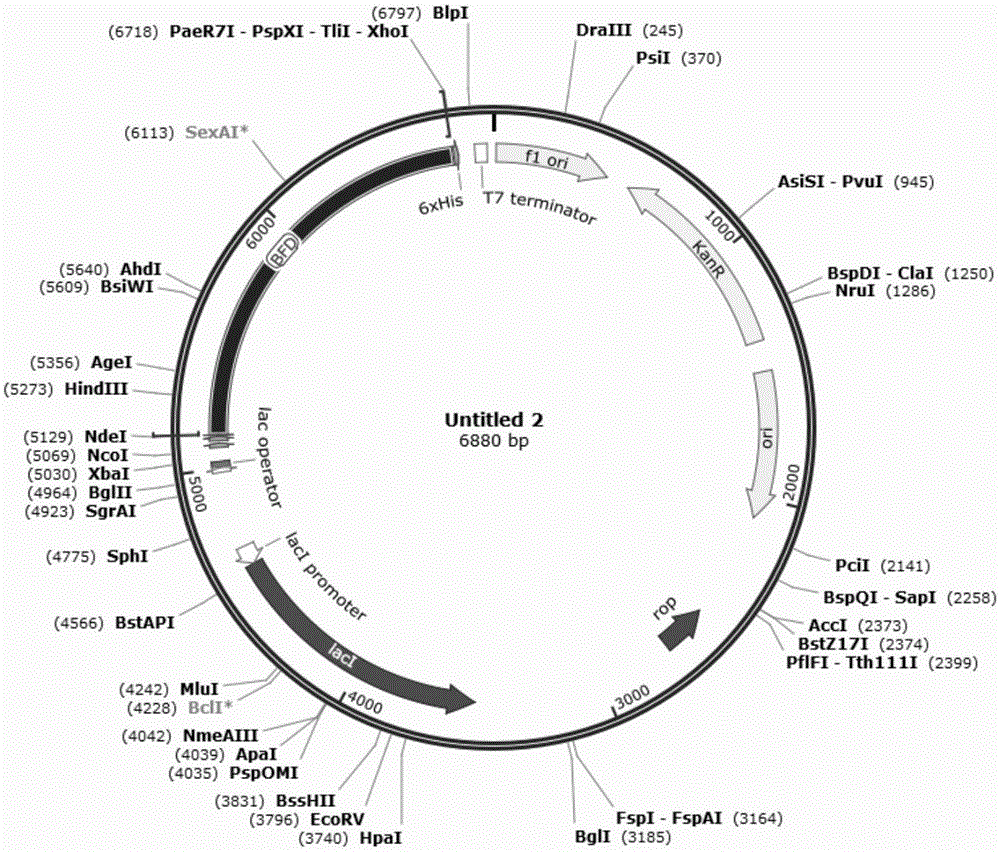
[0037]The gene sequence was directly synthesized in the pET-28a vector (Novagen, Kan + ,See figure 1 ), located between the restriction sites NdeI and XhoI, the recombinant plasmid was named pET-28a-bfd.
Embodiment 2
[0038] The expression of embodiment 2 gene
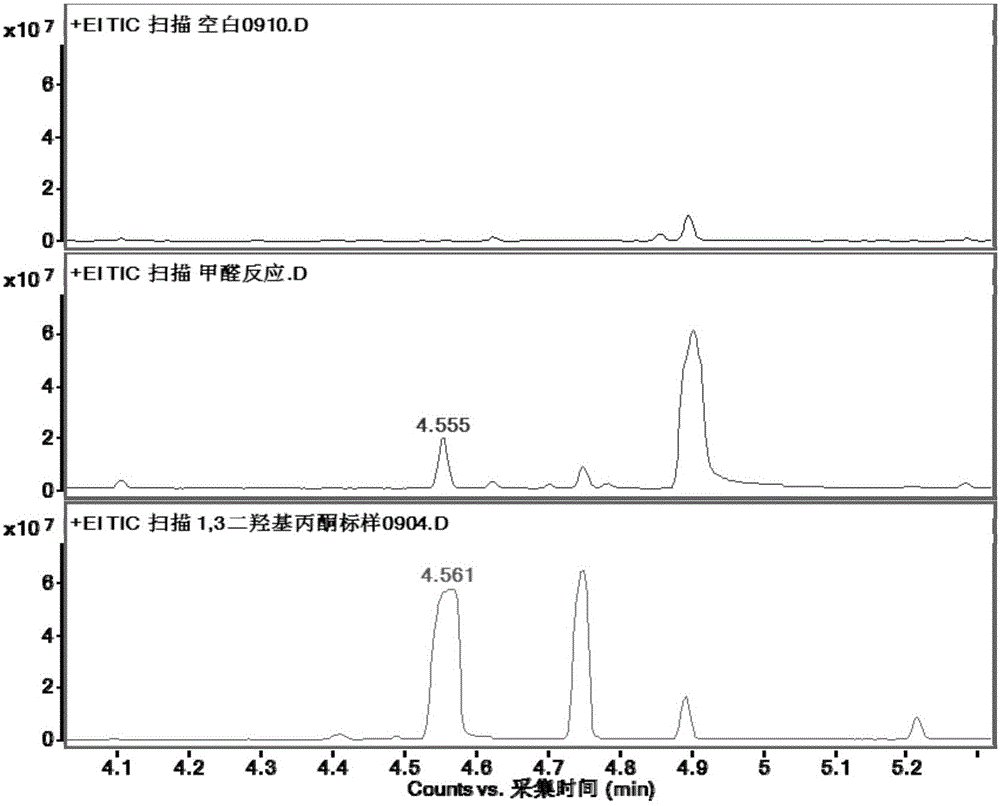
[0039] In order to detect the activity of BFD enzyme in vitro, the enzyme was expressed and purified exogenously in Escherichia coli.
[0040] (1) The Escherichia coli expression recombinant plasmid pET-28a-bfd was transferred into E.coliBL21(DE3) to obtain the recombinant bacteria. Positive clones were screened using kanamycin-resistant plates (Kan + , 100mg / mL), cultivate overnight at 37°C;
[0041] (2) pick a single clone into 5mL LB liquid medium (Kan + , 100mg / mL), cultured at 37℃, 220r / min until OD 600 It is 0.6-0.8. The bacterium liquid in 5mLLB culture medium is transferred in the 800mL2YT culture medium (Kan + , 100mg / mL), cultured at 37℃, 220rpm to OD 600 When the temperature is 0.6-0.8, cool down to 16°C, add IPTG to a final concentration of 0.5mM, and induce expression for 16h;
[0042] (3) The above-mentioned cultured bacteria liquid is collected in the bacteria collection bottle, and centrifuged at 5500r / min for...
Embodiment 3
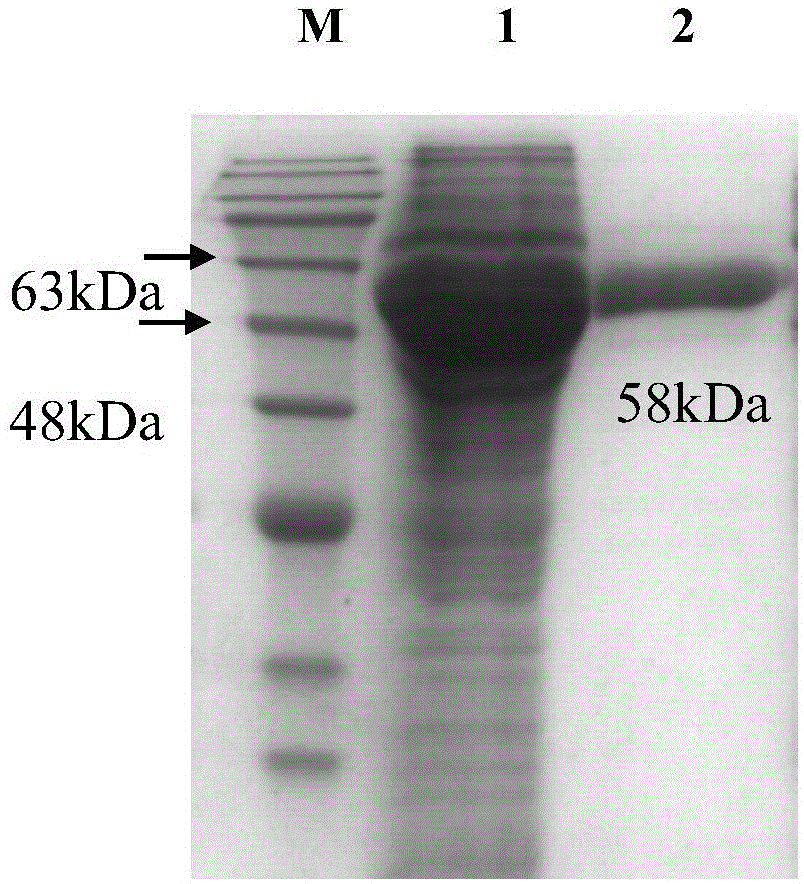
[0044] Example 3 protein purification
[0045] (1) Bacteria destruction: use a high-pressure and low-temperature crusher to destroy bacteria twice at a pressure of 1200 bar and 4°C. Centrifuge at 4°C and 10000r / min for 45min, take the precipitate and supernatant after centrifugation, and prepare samples;
[0046] (2) Purification: the supernatant was suction-filtered through a 0.45 μm microporous membrane, and then purified by nickel affinity chromatography. The specific steps were as follows:
[0047] a: column balance: before hanging the supernatant, first use ddH 2 O washes 2 column volumes, then equilibrates 1 column volume of Ni affinity chromatography column with protein buffer;
[0048] b: Sample loading: slowly pass the supernatant through the Ni affinity chromatography column at a flow rate of 0.5mL / min, and repeat again;
[0049] c: Elution of impurity proteins: Rinse 1 column volume with protein buffer, then use 50mL protein buffer containing 50mM imidazole to el...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com