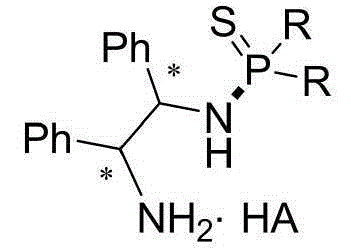
N-(1, 2-diphenyl-2-amino)-thiophosphoramide salt and application thereof
A thiophosphoramide and diphenyl technology, which is applied to N--thiophosphoramide salts and their application fields as pesticides, can solve the problems of being easily oxidized and limited use, and achieve enhanced stability and good chemical stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
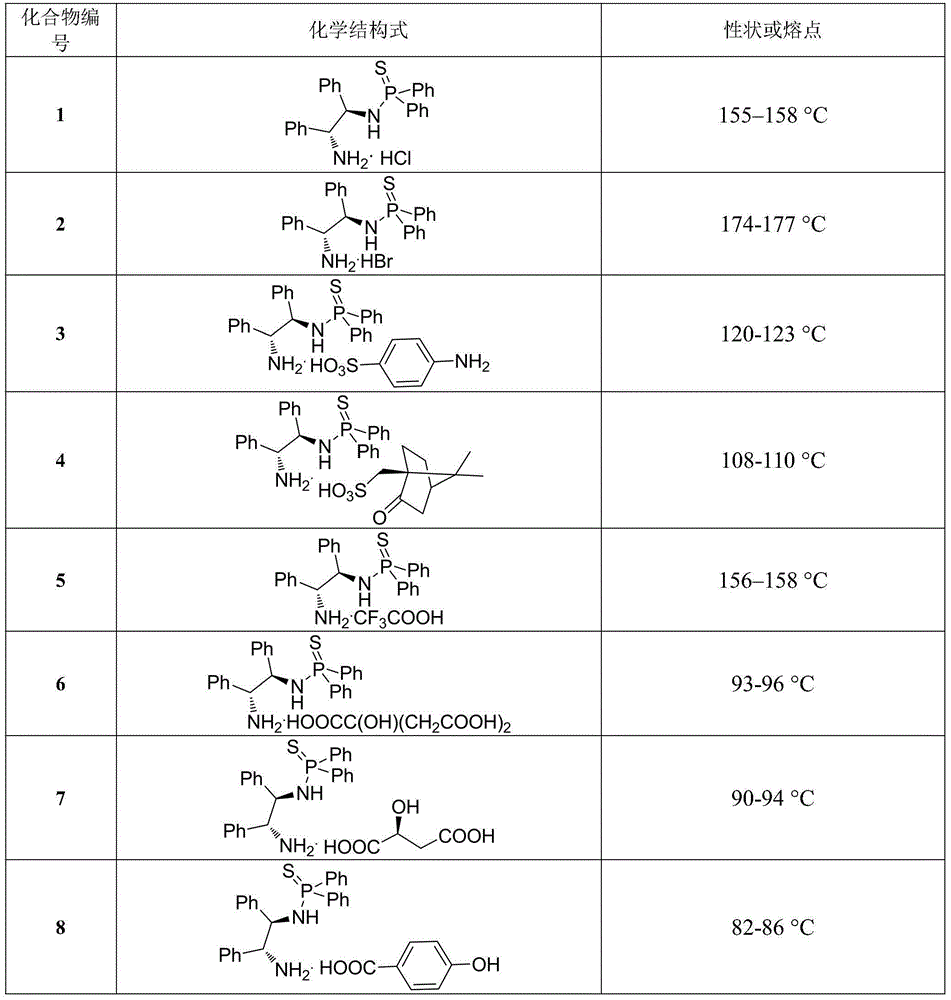
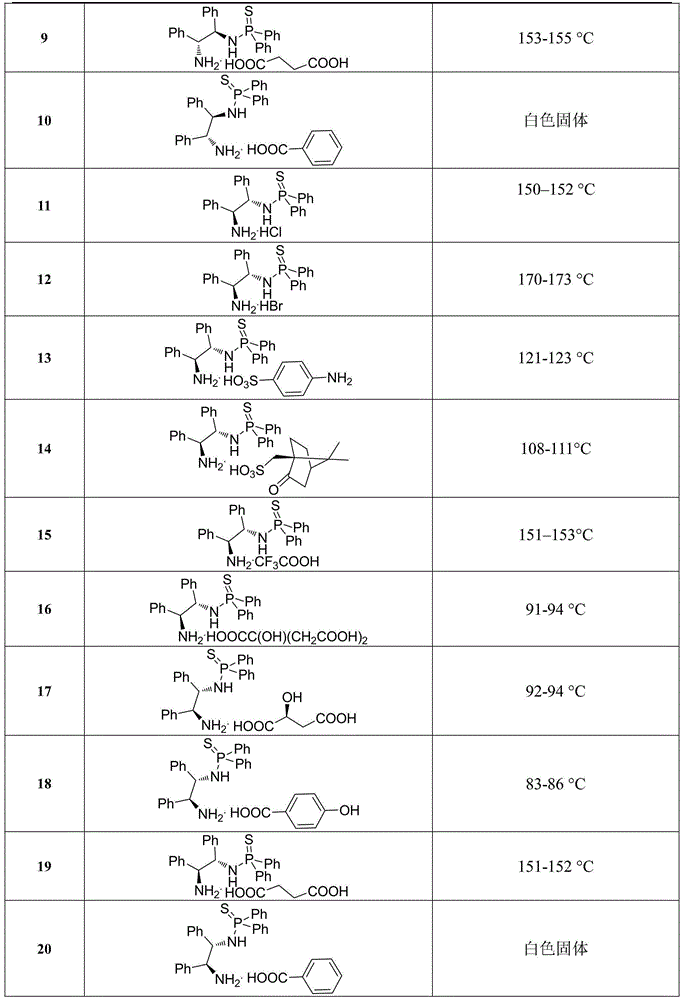
[0019] Example 1. The chemical structural formulas and physical constants of some N-(1,2-diphenyl-2-amino)-thiophosphoramide salts are shown in Table 1:
[0020] Table 1. Some chemical structural formulas and physical constants of N-(1,2-diphenyl-2-amino)-thiophosphoramide salts
[0021]
[0022]
[0023]
[0024] Compared with the known parent compound N-(1R,2R)-(1,2-diphenyl-2-amino)-P,P-diphenylphosphorothioate, the above-mentioned preferred compound has outstanding advantages, which are specifically shown in : (1) The stability of light and heat is significantly enhanced. Under the same conditions, it is irradiated with a fluorescent lamp or controlled at 80°C for 24 hours and then qualitatively detected by NMR. The above compounds do not change, while the control sample N-(1R,2R)-( 1,2-Diphenyl-2-amino)-P,P-diphenylphosphorothioate has been mostly decomposed. (2) Enhanced water solubility, the control sample N-(1R,2R)-(1,2-diphenyl-2-amino)-P,P-diphenylthiophosp...
Embodiment 2
[0025] Example 2: Preparation of target compound N-(1R,2R)-(1,2-diphenyl-2-amino)-P,P-diphenylthiophosphoramide hydrochloride (1)
[0026] At 0°C, (1R,2R)-diphenylethylenediamine (2.12g, 0.01mmol) was dissolved in dichloromethane (10mL), triethylamine (1.4mL) was added, stirred in the reactor, Then diphenylphosphorylthiochloride (0.252g, 0.01mmol) was dissolved in dichloromethane (30mL), added dropwise to the reaction system with a constant pressure dropping funnel, and then detected by TLC. After phosphorus oxychloride disappears, vacuum precipitation, column chromatography purification, the obtained product N-(1R,2R)-(1,2-diphenyl-2-amino)-P,P-diphenylthiophosphoramide . White solid with a yield of 81% and a melting point of 118-120°C. [α] 2 D 0 –23.4 (c1.0, CHCl 3 ); 1 HNMR (CDCl 3 ,400MHz):1.80(s,2H),4.19(t,J=6.8Hz,1H),4.25(d,J=5.6Hz,1H),4.44-4.51(m,1H),7.05-7.08(m, 2H),7.11-7.15(m,3H),7.18-7.22(m,2H),7.24-7.38(m,9H),7.58-7.65(m,4H);31 PNMR (CDCl 3 ,161.7MHz):59....
Embodiment 3
[0028] Example 3: Preparation of target compound N-(1R,2R)-(1,2-diphenyl-2-amino)-P,P-diphenylthiophosphoramide hydrobromide (2)
[0029] Dissolve N-(1R,2R)-(1,2-diphenyl-2-amino)-P,P-diphenylthiophosphoramide (1mmol) in chloroform (10mL) and methanol (10mL) Hydrogen bromide solution (48%, 1 mL) was added dropwise to the mixed solution, and reacted at room temperature for 3 hours, then anhydrous sodium sulfate was added to dry it, and the corresponding salt was obtained by vacuum precipitation. White solid with a yield of 79% and a melting point of 174-177°C. 1 HNMR (CDCl 3 ,400MHz):4.70-4.79(m,1H),4.89(s,1H),5.76(t,J=10.8Hz,1H),6.95-6.97(m,2H),7.07-7.23(m,10H), 7.33-7.37(m,1H),7.44-7.50(m,3H),7.57-7.62(m,2H),7.89-7.94(m,2H),8.70(s,3H); 31 PNMR (CDCl 3 ,161.7MHz):64.16; 13 CNMR (CDCl 3 ,100.6MHz):59.7(d,J=2.8Hz),60.0,127.8,127.9,128.0,128.1,128.2,128.4,128.6,128.7,128.9,129.3,131.3,131.4,131.6,131.75,131.018,132. , 132.28, 132.31, 132.33, 132.6, 133.1, 134.2, 138.4 (d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com