Erlotinib preparation method suitable for industrial production
A technology of erlotinib and erlotinib hydrochloride, applied in the field of synthesis of N--6,7-di-4-aminoquinazoline and its hydrochloride, can solve hydroxyl protection and deprotection reactions The problems such as cumbersome process and long synthetic route steps can achieve the effect of avoiding the increase of reaction impurities, saving the use of solvents and improving product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
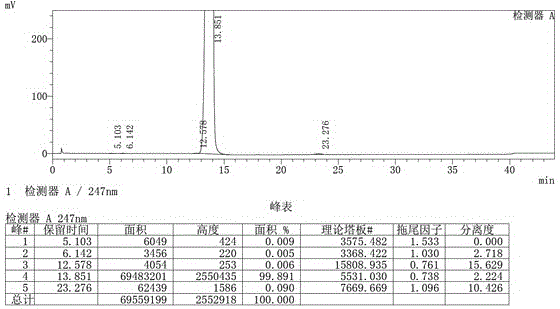
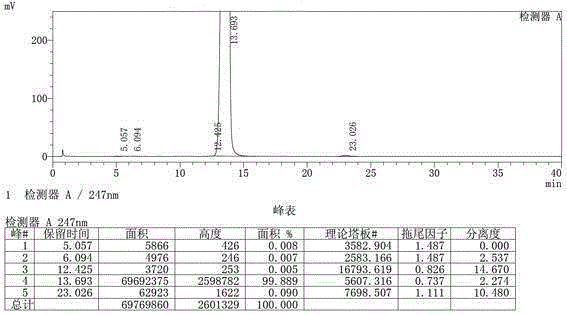
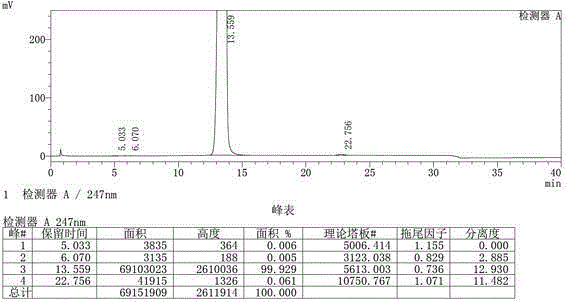
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of 4,5-bis(2-methoxyethoxy)-2-aminophenylacetonitrile
[0034] Add 4.00 kg of 4,5-bis(2-methoxyethoxy)-2-nitrophenylacetonitrile and 80.0 kg of drinking water into the reactor, and stir for 20 minutes. Control the reaction temperature at 45-55°C, and add 7.05 kg of sodium dithionite in 10 batches within 5 hours. After the addition was complete, the reaction was continued at 45-55°C, and the reaction was monitored by TLC. After the reaction was complete, the temperature was raised to 65-75°C, and concentrated hydrochloric acid was added dropwise. After the addition, keep the reaction for 2 hours, then cool to 19-25 °C, adjust the pH to 8-9 with ammonia water, stir and crystallize for 2 hours, filter, and wash with drinking water 3.0kg×2. The wet product was air-dried at 65-75°C to obtain 3.45 kg of 4,5-bis(2-methoxyethoxy)-2-aminophenylacetonitrile. (Yield: 96%, HPLC: 95%).
Embodiment 2
[0035] Embodiment 2: ( E Preparation of )-N'-(2-cyano-4,5-bis(2-methoxyethoxy)phenyl)-N,N-dimethylformamidine
[0036]2.34kg of 4,5-bis(2-methoxyethoxy)-2-aminophenylacetonitrile, 2.09kg of N,N-dimethylformamide dimethyl acetal and 4.68kg of N,N-dimethyl Formamide was added into a 20L reaction flask, heated to reflux, and the reaction was stopped after 3 hours. Distillation under reduced pressure gave 2.82 kg of oil, which was directly used in the next reaction without further treatment.
Embodiment 3
[0037] Embodiment 3: the synthesis of erlotinib
[0038] 2.82kg ( E )-N'-(2-cyano-4,5-bis(2-methoxyethoxy)phenyl)-N,N-dimethylformamidine, 1.03kg m-aminophenylacetylene and 8.61kg ice Acetic acid was added in the 20L reaction flask, heated to reflux, and stopped heating after the reaction was complete (monitored by TLC). Cool to 15-25°C, pour the reaction solution into 50.0kg of ice water with stirring, and then add 20.0kg of ethyl acetate. Under stirring at 15-25°C, adjust the pH to 8-9 with ammonia water, a large amount of solids precipitated, stirred for 2 hours, filtered, and the filter cake was air-dried at 60°C to obtain 2.80kg of crude product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com