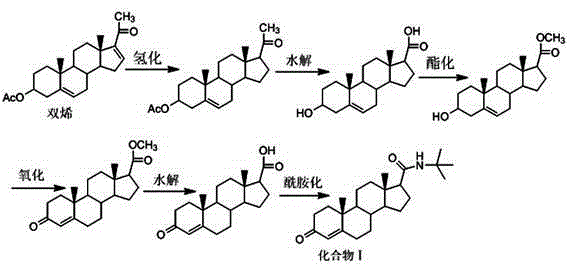
Preparation method for finasteride intermediate
A technology of finasteride and intermediates, which is applied in the field of preparation of finasteride intermediates, can solve the problems of high cost, large pollution, long route and the like, and achieves the effects of low cost, high yield and abundant resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
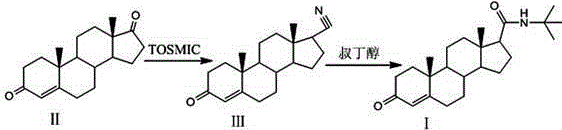
Embodiment 1
[0022] (1) Cyanide
[0023] Under the protection of nitrogen, add 20g of compound II and 40g of potassium tert-butoxide into 800g of ethylene glycol dimethyl ether and 240g of tert-butanol, and cool to -10°C while stirring; slowly add 30g of p-toluenesulfonylmethane After adding isocyanide, heat up to 20°C, stir for 4h, TLC until the raw material reacts completely; analyze with ice water, filter with suction to obtain an off-white solid, wash with water until neutral, dry at 55°C to constant weight, and obtain 18.5g of compound Ⅲ is 3-oxo-4-en-17-cyano;
[0024] (2) Amidation
[0025] Add 18.5g of compound III 3-oxo-4-en-17-cyano into the reaction flask, add 55.5g of glacial acetic acid, 27.8g of tert-butanol, stir well, slowly add 18.5g of sulfuric acid dropwise, and react at a temperature of 10°C for 15h. TLC monitored until the reaction was complete. Add 22.2g of sodium carbonate to terminate the reaction, add 278ml of water for water analysis, filter, wash the filter ca...
Embodiment 2
[0027] (1) Cyanide
[0028] Under the protection of nitrogen, add 50g of compound II and 150g of potassium tert-butoxide into 2250g of ethylene glycol dimethyl ether and 750g of tert-butanol, and cool to 0°C while stirring; slowly add 100g of p-toluenesulfonylmethyl After adding isocyanide, raise the temperature to 25°C, stir for 3 hours, TLC until the reaction of the raw materials is complete, analyze with ice water, filter with suction to obtain an off-white solid, wash with water until neutral, and dry at 60°C to constant weight to obtain 46 g of compound III. 3-oxo-4-en-17-cyano;
[0029] (2) Amidation
[0030] Add 46g of compound III, 3-oxo-4-ene-17-cyano, into the reaction flask, add 230g of glacial acetic acid, 92g of tert-butanol, stir well, slowly add 69g of sulfuric acid dropwise, and react at a temperature of 15°C for 12h, monitored by TLC until the reaction is complete. 82.2 g of sodium carbonate was added to terminate the reaction. Add 920ml of water for water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com