Gelatin-free freeze-drying stabilizer used for human Japanese encephalitis vaccine
A technology of live attenuated vaccine and freeze-dried stabilizer, which is applied in the field of vaccine preparations and achieves the effects of good protection, low irritation and good use safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1. Formula and preparation method of freeze-dried stabilizer
[0023] Screening and optimizing the suitable formula of gelatin-free freeze-drying stabilizer for live attenuated Japanese encephalitis vaccine for human use, which contains salt, sugar and other ingredients, and is prepared by dissolving in water for injection.
[0024]
[0025] Weigh the ingredients in the above prescription in turn, dissolve them in water for injection, and adjust the pH to 6.5-7.8 after all the ingredients are dissolved.
[0026] The stock solution of live attenuated encephalitis Japanese encephalitis vaccine and the freeze-dried stabilizer are mixed according to a certain volume, and the preparation time is not more than 4 hours, and stirred until uniform.
Embodiment 2
[0027] Embodiment 2. Optimization of freeze-drying curve program
[0028] Optimized the time control from pre-freezing to sublimation drying and finally analytical drying. The freeze-drying effect of the finished product is better, and the formability is better. Turn on the freeze dryer, and when the temperature of the plate layer reaches -30°C to -40°C, the product is allowed to enter the cabinet and keep it until the product enters the cabinet. After the product is put into the cabinet and the cabinet door is closed and confirmed, the product is accelerated to refrigerate, so that all the products in the cabinet are completely frozen (keep below -40°C for more than 2 hours). Create a low-pressure environment (below 10Pa) for the box, so that the product can be dried and vacuumed at low temperature and low pressure. The first stage of drying: Slowly heat the product to -10°C to 0°C and keep it until the appearance of the product is shaped into a light yellow loose body with...
Embodiment 3
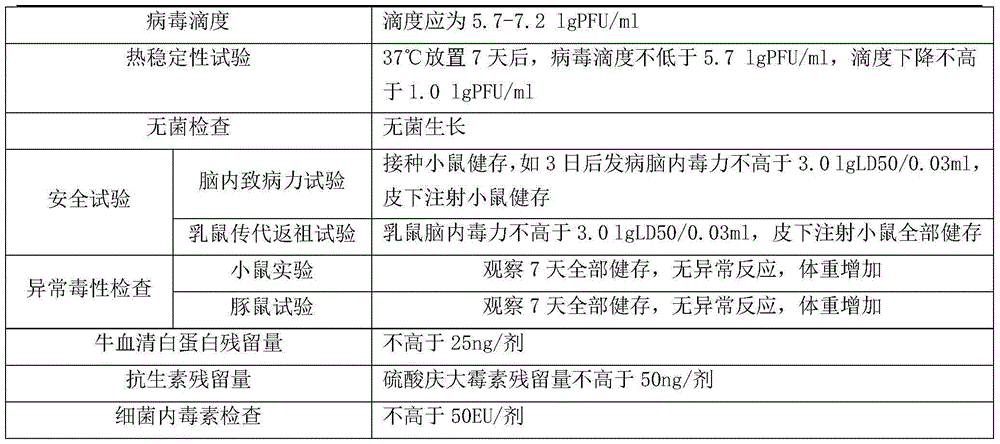
[0029] Embodiment 3. Product quality detection after freeze-drying
[0030] According to the relevant regulations of the third part of the Pharmacopoeia of the People's Republic of China, all relevant items are tested.
[0031]
[0032]
[0033] The test results showed that all indicators of the vaccine products freeze-dried without gelatin freeze-drying stabilizer were in line with national regulations and enterprise standards.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com