Compound dipyridamole and aspirin tablet and preparation method thereof
A technology for dipyridamole aspirin and dipyridamole tablet cores is applied in compound tablets and their preparation, and in the field of compound dipyridamole aspirin tablets and their preparation, and can solve gastric wall mucosal damage, unfavorable long-term medication, unfavorable Amplify production and other issues to achieve the effects of improving solubility and absorption, improving bioavailability, and improving placement stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
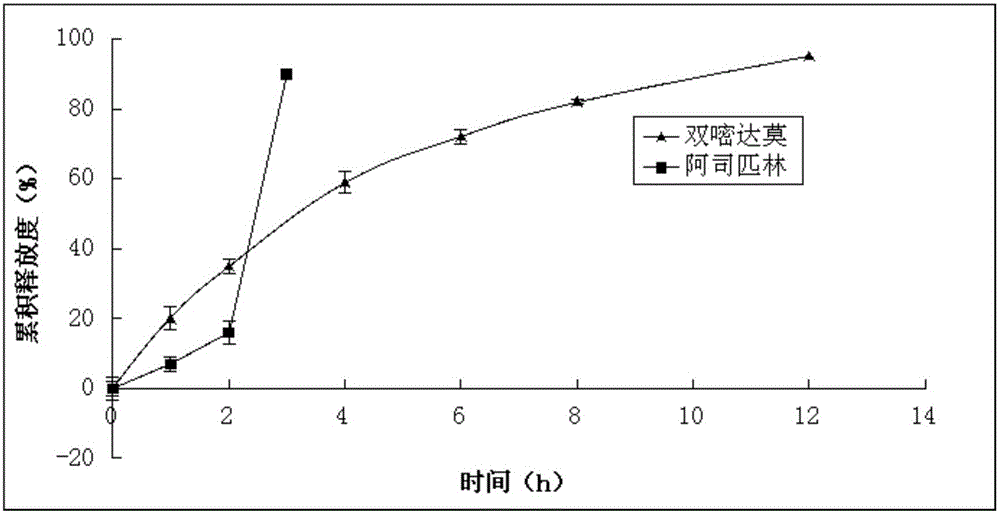
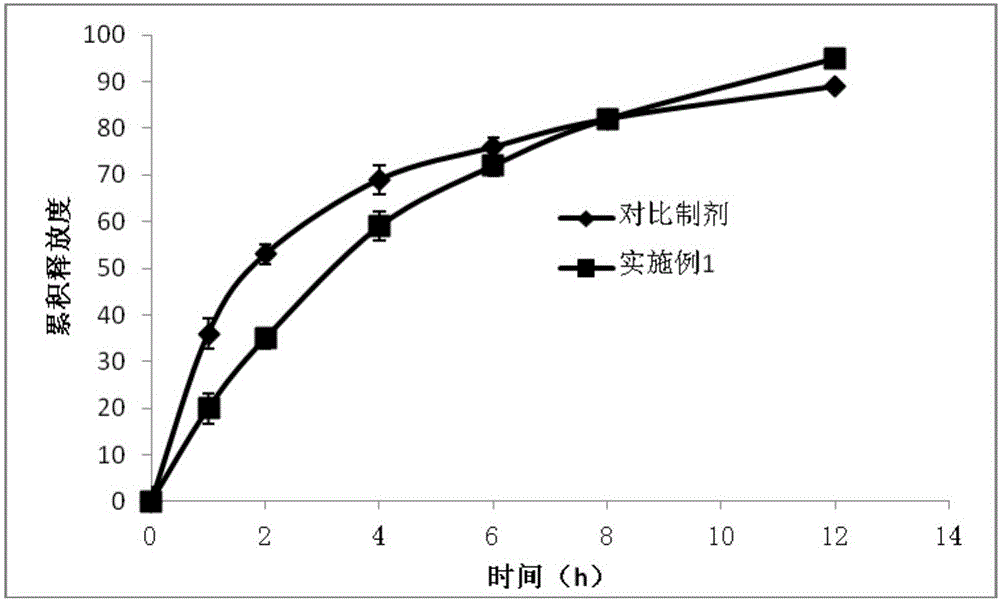
Image
Examples
Embodiment 1
[0053] Example 1 Compound dipyridamole aspirin tablet of the present invention (specification: 200mg / 25mg, 1000 tablets)
[0054] Prescription composition:
[0055] (1) Dipyridamole extended-release part
[0056]
[0057] (2) Aspirin part
[0058]
[0059]
[0060] (3) The outer part of the tablet
[0061] HPMC17B type coating powder 15g.
[0062] The preparation method comprises the steps of:
[0063] (1) Preparation of dipyridamole tablet core
[0064] A, get the dipyridamole of recipe quantity, acidic additive (1), 20% sustained-release retardant and cross 80 mesh sieves and mix homogeneously, and material is for subsequent use;
[0065] B, remaining 80% slow-release retarder is mixed with slow-release retarder weight percent is 5% 70% ethanol aqueous solution, and solution is for subsequent use;
[0066] C. Mix the material obtained in step A with the solution obtained in step B to make a soft material. After passing through a 20-mesh sieve, place it in an o...
Embodiment 2
[0076] Example 2 Compound dipyridamole aspirin tablet of the present invention (specification: 200mg / 25mg, 1000 tablets)
[0077] Prescription composition:
[0078] (1) Dipyridamole extended-release part
[0079]
[0080]
[0081] (2) Aspirin part
[0082]
[0083] (3) The outer part of the tablet
[0084] HPMC17B type coating powder 18g
[0085] The preparation method comprises the steps of:
[0086] (1) Preparation of dipyridamole tablet core
[0087] A, get the dipyridamole of recipe quantity, acidic additive (1), 20% sustained-release retardant and cross 80 mesh sieves and mix homogeneously, and material is for subsequent use;
[0088] B, remaining 80% slow-release retarder is mixed with slow-release retarder weight percentage is 10% 70% ethanol aqueous solution, and solution is for subsequent use;
[0089] C. Mix the material obtained in step A with the solution obtained in step B to make a soft material. After passing through a 20-mesh sieve, place it in a...
Embodiment 3
[0099] Example 3 Compound dipyridamole aspirin tablet of the present invention (specification: 200mg / 25mg, 1000 tablets)
[0100] Prescription composition:
[0101] (1) Dipyridamole part
[0102]
[0103] (2) Aspirin part
[0104]
[0105] (3) The outer part of the tablet
[0106] HPMC17B type coating powder 12g;
[0107] The preparation method comprises the steps of:
[0108] (1) Preparation of dipyridamole tablet core
[0109] A, get the dipyridamole of recipe quantity, acidic additive (1), 20% sustained-release retardant and cross 80 mesh sieves and mix homogeneously, and material is for subsequent use;
[0110] B, remaining 80% slow-release retarder is mixed with slow-release retarder weight percent is 1% 70% ethanol aqueous solution, and solution is for subsequent use;
[0111] C. Mix the material obtained in step A with the solution obtained in step B to make a soft material. After passing through a 20-mesh sieve, place it in an oven and dry it at 40°C for 4 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com