Preparation method of chitobiose derivative crosslinking fibers
A chitosan derivative, chitosan technology, applied in the direction of fiber chemical characteristics, textiles and papermaking, etc., can solve problems such as taking a long time, achieve the effect of easy operation, high mechanical strength, and expand the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
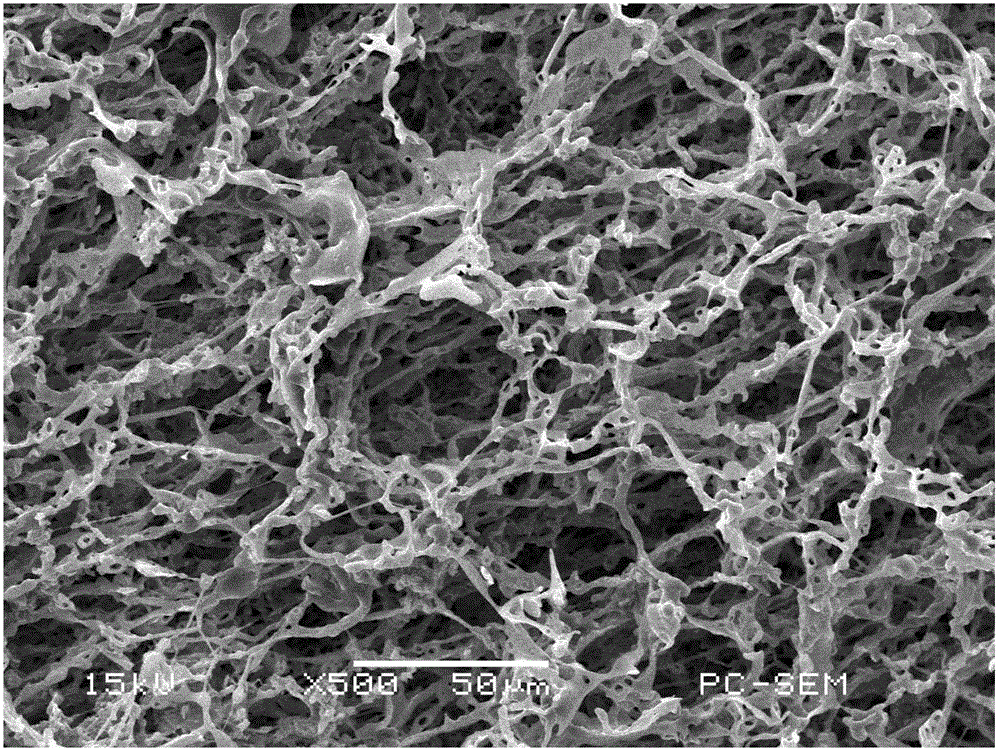
Image
Examples
Embodiment 1
[0025] (1) Dissolve 2g of chitosan (deacetylation degree DP=85%, weight average molecular weight Mw=3000) in dilute acetic acid solution, add glycidyl methacrylate, heat and stir for 12h, after the reaction is completed, after-treatment A relatively pure photopolymerizable chitosan derivative is obtained.
[0026] (2) Dissolve photopolymerizable chitosan derivatives and photoinitiator 2959 (2959 and photopolymerizable chitosan derivatives=0.1 / 1) in (1) in solvent water (photopolymerizable chitosan The mass ratio of derivatives to water = 1 / 100), frozen at 0°C to freeze and crystallize the water, and use a light intensity of 20mW / cm at 0°C 2 The ultraviolet light source was irradiated for 40 minutes to cross-link, and the solvent water was removed by freeze-drying to obtain a cross-linked chitosan derivative fiber material.
Embodiment 2
[0028] (1) Dissolve 2g of chitosan (deacetylation degree DP=85%, weight average molecular weight Mw=5000) in dilute acetic acid solution, add glycidyl methacrylate, heat and stir for 12h, after the reaction is completed, after-treatment A relatively pure photopolymerizable chitosan derivative is obtained.
[0029] (2) photopolymerizable chitosan derivatives and photoinitiator 8700 (mass ratio=0.001 / 1 of photoinitiator 8700 and photopolymerizable chitosan derivatives) in (1) are dissolved in solvent water (can be The mass ratio of photopolymerized chitosan derivatives to water = 1 / 1000), frozen at -197°C to freeze and crystallize the water, and use a light intensity of 30mW / cm at -197°C 2 The ultraviolet light source is irradiated for 30 minutes to cross-link, and the solvent water is removed by freeze-drying to obtain a cross-linked chitosan derivative fiber material.
Embodiment 3
[0031] (1) Dissolve 2g of chitosan (deacetylation degree DP=88%, weight average molecular weight Mw=8000) in dilute acetic acid solution, add glycidyl methacrylate, heat and stir for 12h, after the reaction is completed, after-treatment A relatively pure photopolymerizable chitosan derivative is obtained.
[0032] (2) photopolymerizable chitosan derivatives and water-soluble thioxanthone photoinitiators (water-soluble thioxanthone photoinitiators and photopolymerizable chitosan derivatives in (1) Mass ratio=0.005 / 1) be dissolved in the mixture of solvent water and acetic acid (mass ratio=5 / 10000 of the mixture of photopolymerizable chitosan derivative and water and acetic acid, the mass ratio of water and acetic acid is 100 / 1 ), frozen at -50°C to freeze and crystallize water and acetic acid, and use a light intensity of 50mW / cm at a low temperature of -20°C 2 The ultraviolet light source is irradiated for 20 minutes for crosslinking, and the solvent water and acetic acid are...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com