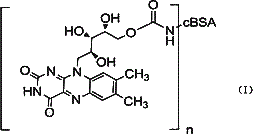
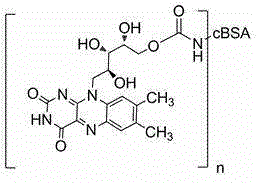
Vitamin B2 conjugate and preparation method and application thereof
A vitamin and conjugate technology, applied in the field of vitamin B2 conjugate and its preparation, can solve problems such as complicated steps, save detection time, and make up for long time-consuming effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Preparation of solution A: vitamin B 2 19.4mg, N,N'-carbonyldiimidazole (CDI) 25.0mg, 4-dimethylaminopyridine (DMAP) 0.6mg dissolved in 4ml dimethylformamide, the reaction produces vitamin B 2 Active intermediate with N,N'-carbonyldiimidazole, spare;
[0042] (2) Preparation of cBSA: Under the condition of 0~4°C, first dissolve 18.0mg of ethylenediamine in 20ml of phosphate buffer solution with a pH of 7.40 and a concentration of 0.01M, adjust the pH to 7.40 with concentrated hydrochloric acid; weigh 1000.0 mgBSA (molecular weight 68,000) and 57.51mg EDC, then added to the ethylenediamine solution, stirred and reacted at 20°C for 2 hours; the reaction solution of ethylenediamine and BSA was buffered with the above-mentioned phosphate buffer at 0~4°C The dialyzed solution was stirred and dialyzed for 70 hours, and then dialyzed with distilled water for 24 hours, and the dialysate was replaced every 6 hours; the dialyzed solution was centrifuged at 13,000 rpm for 15 ...
Embodiment 2
[0048] (1) Preparation of solution A: vitamin B 2 19.4mg, N,N'-carbonyldiimidazole (CDI) 30.0mg, 4-dimethylaminopyridine (DMAP) 1.0mg dissolved in 5ml dimethylformamide, the reaction produces vitamin B 2 Active intermediate with N,N'-carbonyldiimidazole, spare;
[0049] (2) Preparation of cBSA: Under the condition of 0~4°C, first dissolve 18.0mg of ethylenediamine in 20ml of phosphate buffer solution with a pH of 7.40 and a concentration of 0.01M, adjust the pH to 7.40 with concentrated hydrochloric acid; weigh 1000.0 mgBSA (molecular weight 68,000) and 57.51mg EDC, then added to the ethylenediamine solution, stirred and reacted at 20°C for 2 hours; the reaction solution of ethylenediamine and BSA was buffered with the above-mentioned phosphate buffer at 0~4°C The dialyzed solution was stirred and dialyzed for 70 hours, and then dialyzed with distilled water for 30 hours, and the dialysate was replaced every 6 hours; the dialyzed solution was centrifuged at 13,000 rpm for 15 ...
Embodiment 3
[0055] (1) Preparation of solution A: vitamin B 2 19.4mg, N,N'-carbonyldiimidazole (CDI) 35.0mg, 4-dimethylaminopyridine (DMAP) 1.3mg dissolved in 7ml dimethylformamide, the reaction produces vitamin B 2 Active intermediate with N,N'-carbonyldiimidazole, spare.
[0056] (2) Preparation of cBSA: Under the condition of 0~4°C, first dissolve 18.52mg of ethylenediamine in 20ml of phosphate buffer solution with a pH of 7.56 and a concentration of 0.02M, adjust the pH to 7.56 with concentrated hydrochloric acid; weigh 1000.00 mgBSA (molecular weight 68,000) and 57.51mg EDC, then added to the ethylenediamine solution, stirred and reacted at 22°C for 3 hours; the reaction solution of ethylenediamine and BSA was buffered with the above-mentioned phosphate buffer at 0~4°C The dialyzed solution was stirred and dialyzed for 80 hours, and then dialyzed with distilled water for 20 hours, and the dialysate was replaced every 6 hours; the dialyzed solution was centrifuged at 13,000 rpm for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com