Quinacridones-containing organic electroluminescence material and preparation method thereof
A technology of quinacridone and luminescent materials, applied in the field of organic electroluminescent materials, to achieve the effects of high yield, increased planar conjugation, and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
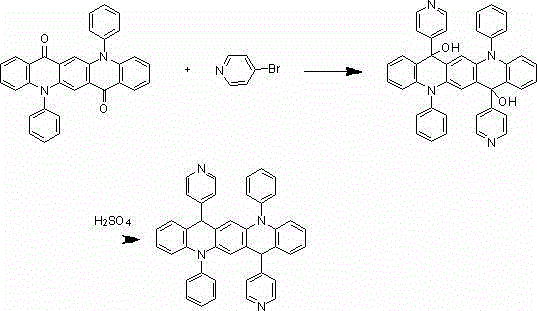
[0022] The synthesis of embodiment 1 compound 001
[0023]
[0024] Under nitrogen protection system, weigh 1.5mmol of bromobenzene, add 300ml of freshly steamed tetrahydrofuran solution, add 1.2mmol of n-butyllithium dropwise at -78°C, react for two hours, then add dropwise 200ml of tetrahydrofuran solution Dissolved 1 mmol of 5,12-diphenyl-5,12-dihydroquinoline[2,3-b]acridine-7,14-dione, after the dropwise addition, react at room temperature for 9 hours under nitrogen protection conditions, Add 100ml of 1M dilute hydrochloric acid, extract 3 times with 100ml of diethyl ether, separate the liquid, concentrate, and recrystallize with dichloromethane:petroleum ether=1:3 to obtain a light yellow 0.80mmol intermediate, which is added to 300ml of concentrated sulfuric acid, at room temperature After reacting for 9 hours, hydrolysis, extraction, liquid separation, and recrystallization, 0.75 mmol of a light yellow final product was obtained with a yield of 75% and a purity of mo...
Embodiment 2
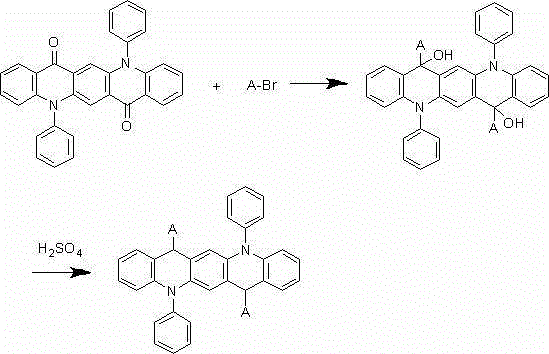
[0025] The synthesis of embodiment 2 compound 002
[0026]
[0027] Under nitrogen protection system, weigh 1.6mmol of 2-bromonaphthalene, add 300ml of freshly steamed tetrahydrofuran solution, and add 1.2mmol of n-butyllithium dropwise at -78°C, react for two hours, then add 200ml of 1 mmol of 5,12-diphenyl-5,12-dihydroquinoline[2,3-b]acridine-7,14-dione dissolved in tetrahydrofuran solution, after the dropwise addition, react at room temperature under nitrogen protection conditions 9 After 1 hour, add 100ml of 1M dilute hydrochloric acid, extract 3 times with 100ml of diethyl ether, separate liquid, concentrate, and recrystallize with dichloromethane:petroleum ether=1:3 to obtain a pale yellow 0.82mmol intermediate, which is added to 300ml of concentrated sulfuric acid , reacted at room temperature for 9 hours, after hydrolysis, extraction, liquid separation, and recrystallization, 0.76 mmol of a light yellow final product was obtained with a yield of 76% and a purity of ...
Embodiment 3
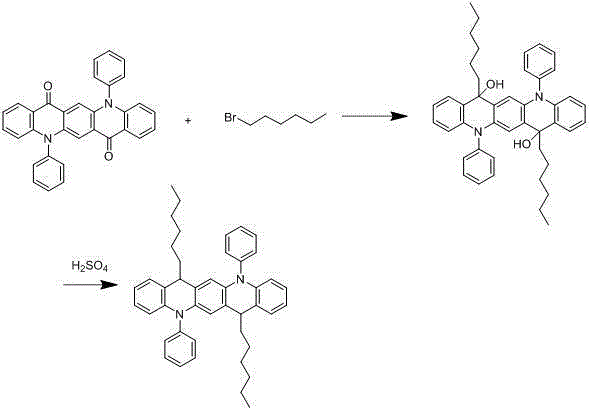
[0028] The synthesis of embodiment 3 compound 003
[0029]
[0030] Under nitrogen protection system, weigh 1.7mmol of hexane bromide, add 300ml of freshly distilled tetrahydrofuran solution, and add 1.2mmol of n-butyllithium dropwise at -78°C, react for two hours, and then add 200ml of tetrahydrofuran solution dropwise. 1 mmol of 5,12-diphenyl-5,12-dihydroquinoline[2,3-b]acridine-7,14-dione dissolved in the solution, after the dropwise addition, react at room temperature for 9 hours under nitrogen protection , add 100ml of 1M dilute hydrochloric acid, extract 3 times with 100ml of diethyl ether, separate liquid, concentrate, recrystallize with dichloromethane:petroleum ether=1:3, obtain light yellow 0.82mmol intermediate, add the intermediate to 300ml concentrated sulfuric acid, After reacting at room temperature for 9 hours, after hydrolysis, extraction, liquid separation and recrystallization, 0.73 mmol of a light yellow final product was obtained with a yield of 73% and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com