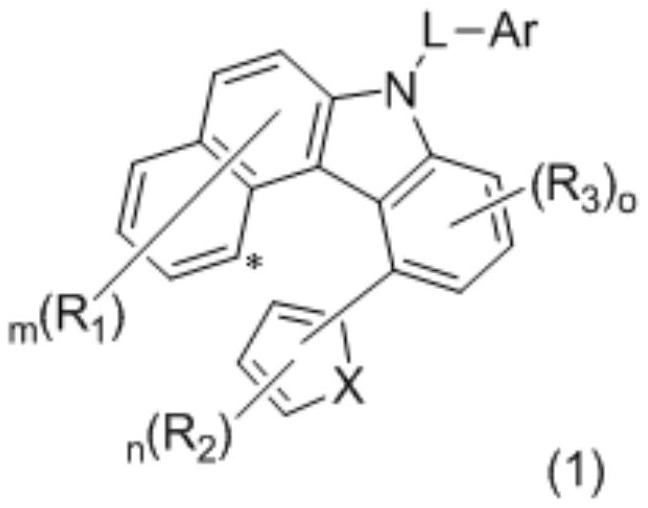
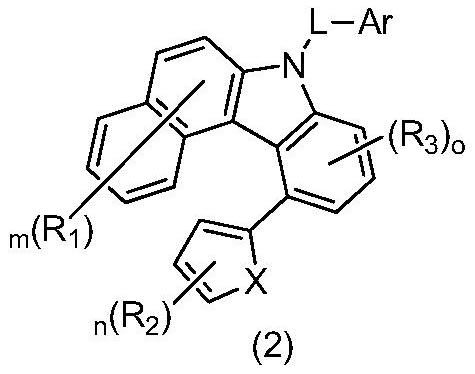
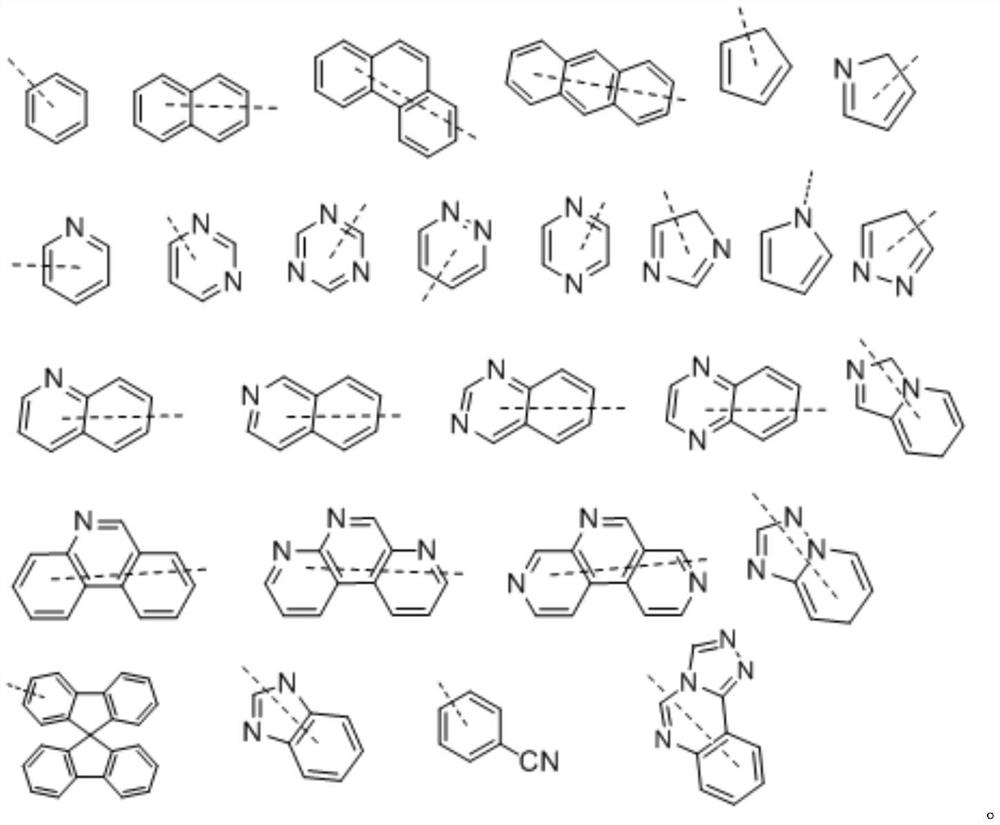
Organic compound for organic electroluminescent device and application thereof
An organic compound, unsubstituted technology, applied in organic chemistry, electrical solid devices, electrical components, etc., to achieve the effects of increased stacking, increased planar conjugation, and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0073] Synthesis of Compound C1
[0074]
[0075] (1) Preparation of compound 1-1
[0076] The compound 1-naphthalene boronic acid (344g, 2mol), 2,3-dichloronitrobenzene (566g, 2mol), potassium carbonate (828g, 3mol) was added into a mixture containing 1,4-dioxane / water (8L / 1L ) in the flask, after replacing the nitrogen with stirring at room temperature, add tetrakistriphenylphosphine palladium (11.5 g, 10 mmol), replace the nitrogen three times after the addition, stir and reflux for 5 hours, and monitor the end of the reaction by TLC. Remove 1,4-dioxane by rotary evaporation under reduced pressure, add dichloromethane to dissolve, and separate the layers. The organic phase was dried with anhydrous sodium sulfate, filtered, 5 L of methanol was added and stirred at room temperature overnight after the solvent was removed under reduced pressure. A brown solid was precipitated by filtration and dried in air to obtain compound 1-1 (526 g, yield 93%).
[0077] (2) Preparatio...
Synthetic example 2
[0086] Synthesis of Compound C22
[0087]
[0088] (1) Preparation of compound 2-1
[0089] Add compound 1-3 (20g, 58.3mmol), 2-bromobenzofuran (11.4g, 58.3mmol), potassium carbonate (24.1g, 175mmol) into a flask containing toluene / ethanol / water 250mL / 50mL / 50mL, After replacing the nitrogen with stirring at room temperature, tetrakistriphenylphosphine palladium (0.7 g, 0.6 mmol) was added. After the addition, the nitrogen was replaced three times, and the reaction was stirred and refluxed for 5 hours, and the end point of the reaction was monitored by TLC. Cool to room temperature and separate the liquids. The organic phase was extracted with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, the solvent was removed under reduced pressure and purified by column chromatography to obtain brown compound 2-1 (11.8 g, yield 61%).
[0090] (2) Preparation of compound 2-2
[0091] The compound 1-chloro-2-phenyl-4-(4-biphenyl)-1,3,5-t...
Synthetic example 3
[0095] Synthesis of Compound C37
[0096]
[0097] (1) Preparation of compound 3-1
[0098] Add compound 1-3 (15.4g, 45mmol), 2-bromothiophene (7.3g, 45mmol), potassium carbonate (18.6g, 135mmol) into a flask containing toluene / ethanol / water 250mL / 50mL / 50mL, stir at room temperature After replacing the nitrogen, tetrakistriphenylphosphine palladium (520 mg, 0.45 mmol) was added. After the addition, the nitrogen was replaced three times, and the mixture was stirred and refluxed for 7 hours. The end point of the reaction was monitored by TLC. Cool to room temperature and separate the liquids. The organic phase was extracted with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, the solvent was removed under reduced pressure and purified by column chromatography to obtain compound 3-1 (8.7 g, yield 65%).
[0099] (2) Preparation of compound 3-2
[0100] The compound 1-chloro-4-phenylquinazoline (24g, 0.1mol), 3-chloro-3-biphenyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com