Vaccine for preventing brucellosis by means of ophthalmic drug delivery
A technology for brucellosis and ocular drug delivery, which is applied in the direction of antibacterial drugs and bacterial antigen components, and can solve problems such as bacteria excretion and pregnant cow abortion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] ——Comparison test of diluents of live brucellosis vaccine
[0054] The vaccine is diluted with the following 4 kinds of diluents:
[0055] (1) Sterile physiological saline;
[0056] (2) Phosphate buffer;
[0057] (3) Sterile physiological saline containing color indicator;
[0058] (4) Phosphate buffer containing color indicator.
[0059] Trypsin is used in the diluted vaccine The agar plate was used to count viable bacteria (colony forming units, CFU). The vaccine was placed at room temperature, and samples were taken for 1 hour, 2 hours, 3 hours, 4 hours, and 5 hours respectively, and the viable bacteria were counted on a tryptic agar plate. The results are shown in Table 1.
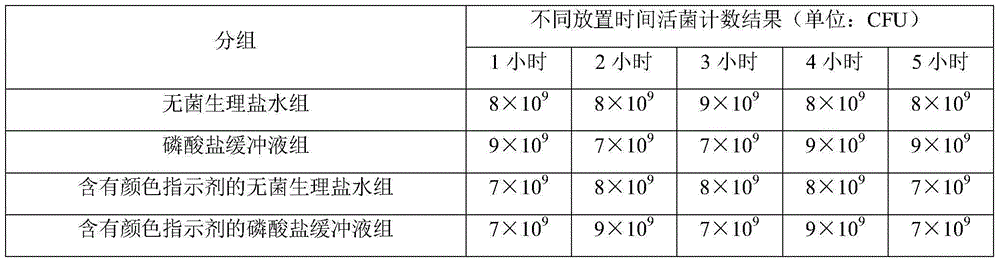
[0060] Table 1 Counting results of live bacteria at different storage time
[0061]
[0062] The results in Table 1 show that the vaccine was diluted with the above 4 kinds of diluents and placed at room temperature for 4 hours. The number of live bacteria before the vaccine was placed and the number of l...
Embodiment 2
[0064] ——Vaccine use
[0065] (1) Pour the diluent into the vaccine bottle, suspend and mix;
[0066] (2) Use an eye dropper (or syringe) to drip the vaccine into the orbit of the animal to be immunized, and the vaccine liquid does not flow out of the animal's orbit as a successful immunization.
Embodiment 3
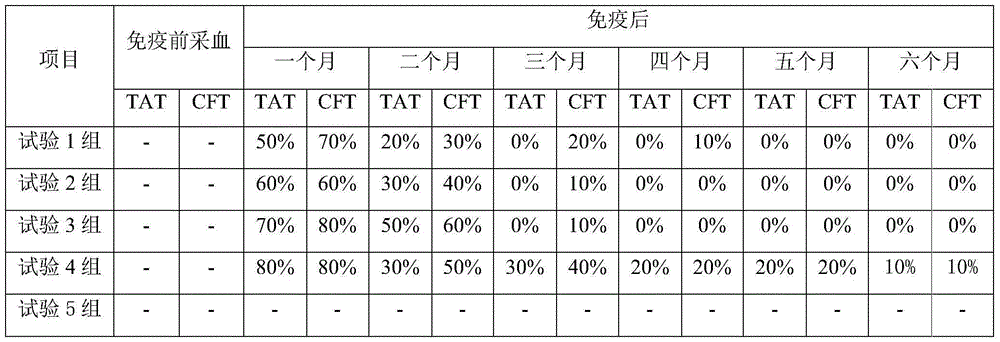
[0068] -Immunization program
[0069] (1) One part of eye immunity;
[0070] (2) One dose of ocular immunization, one dose of immunization with the same method at intervals of 3-8 months;
[0071] (3) Subcutaneous injection of a standard dose (6×10 10 ~8×10 10 CFU), one dose of ocular immunization every 3 to 8 months;
[0072] (4) One dose of ocular immunization, with an interval of 3-8 months, subcutaneous injection of a standard dose (6×10 10 ~8×10 10 CFU).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com