Stable glucokinase activator compositions
A technology of composition and alkalizing agent, which is applied in the direction of drug combination, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of particle aggregation, increasing particle size or inducing stabilizers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Preparation of solid stabilized particles
[0026] The stabilized pharmaceutical compositions of the invention comprise solid stabilized particles. Stable solid particles can be prepared as follows: starting from the or a pharmaceutically acceptable salt thereof and a solvent, from which the solvent is then removed to form solid stabilized particles. Particle suspensions include microsuspensions, i.e., suspensions comprising particles in the micron size range from about 1 μm to about 100 μm, or nanosuspensions, i.e., suspensions comprising particles in the nanometer size range from about 0.5 nm to about 1000 nm , or a mixture of them. In an exemplary embodiment, the particle suspension includes nanoparticles.
[0027] Suitable methods for removing solvent from particle suspensions include, for example, granulation, lyophilization, vacuum drying, oven drying, desiccant drying and spray drying. Suitable solvents for particle suspensions include any solvent generally r...
Embodiment 1
[0094] A nanosuspension of {2-[3-cyclohexyl-3-(trans-4-propoxy-cyclohexyl)-ureido]-thiazol-5-ylsulfanyl}-acetic acid was prepared using the method described above, wherein The polymer stabilizer is hydroxypropyl methylcellulose (HPMC) and the surfactant stabilizer is sodium lauryl sulfate (SLS). The resulting nanosuspension has a solids content of 10%, an average particle size of 225.6 nm, and a particle size of 0.145. Polydispersity index and zeta potential of -57.6 mV.
[0095] The physical stability of the nanosuspensions is shown in Table 1 below, no aggregation was observed after storage at room temperature for 6-48 hours and at 5°C for 1.5 months.
[0096] Table 1: Physical Stability of Nanoparticle Suspensions
[0097] time / temperature
Average particle size (nm)
0
225.6
6h, RT
223.1
24h, RT
230.9
48h, RT
229.4
1.5 months, 5℃
226.0
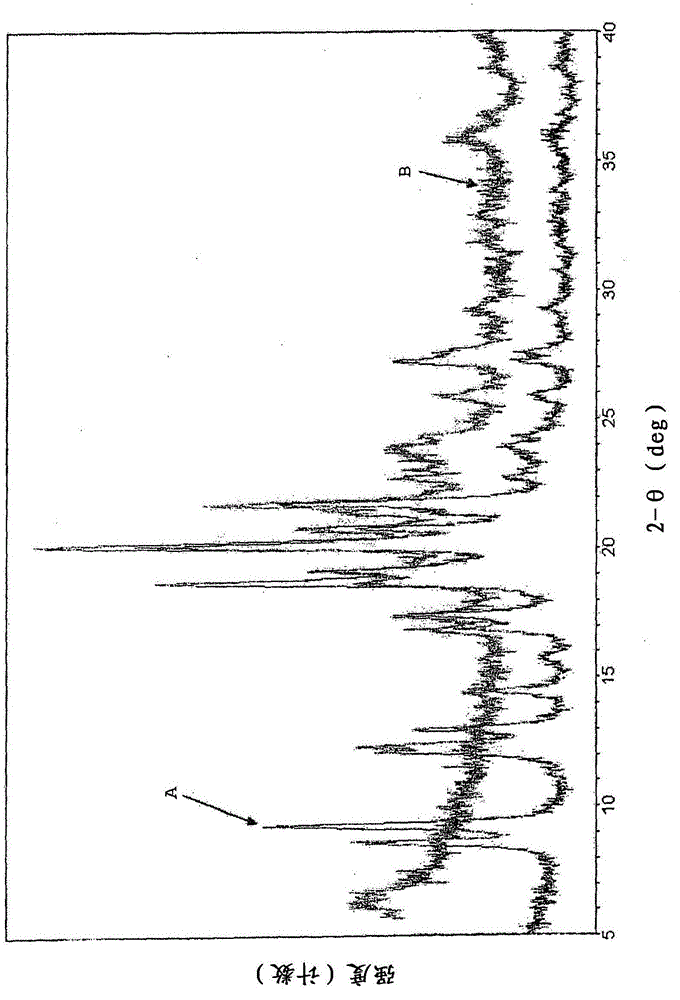
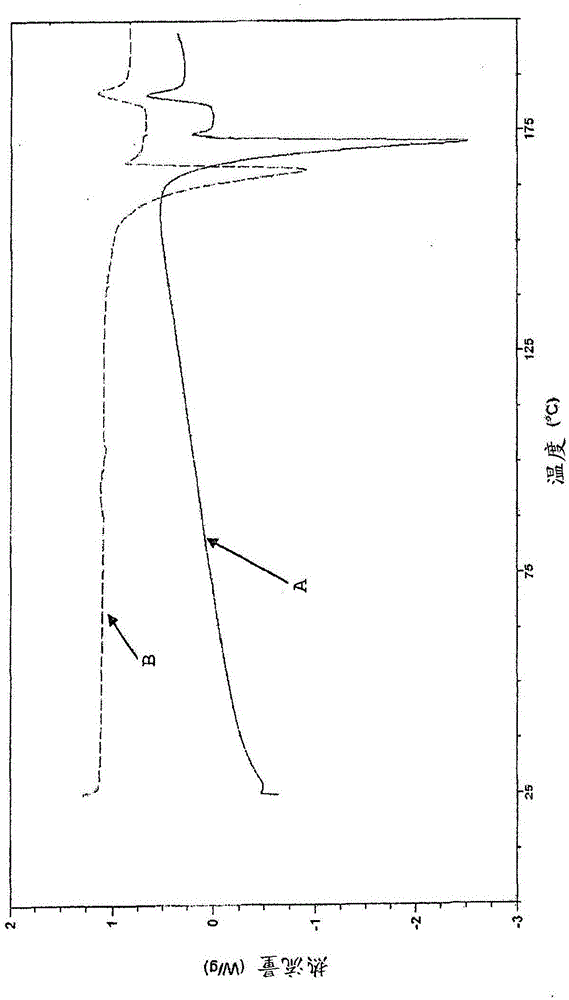
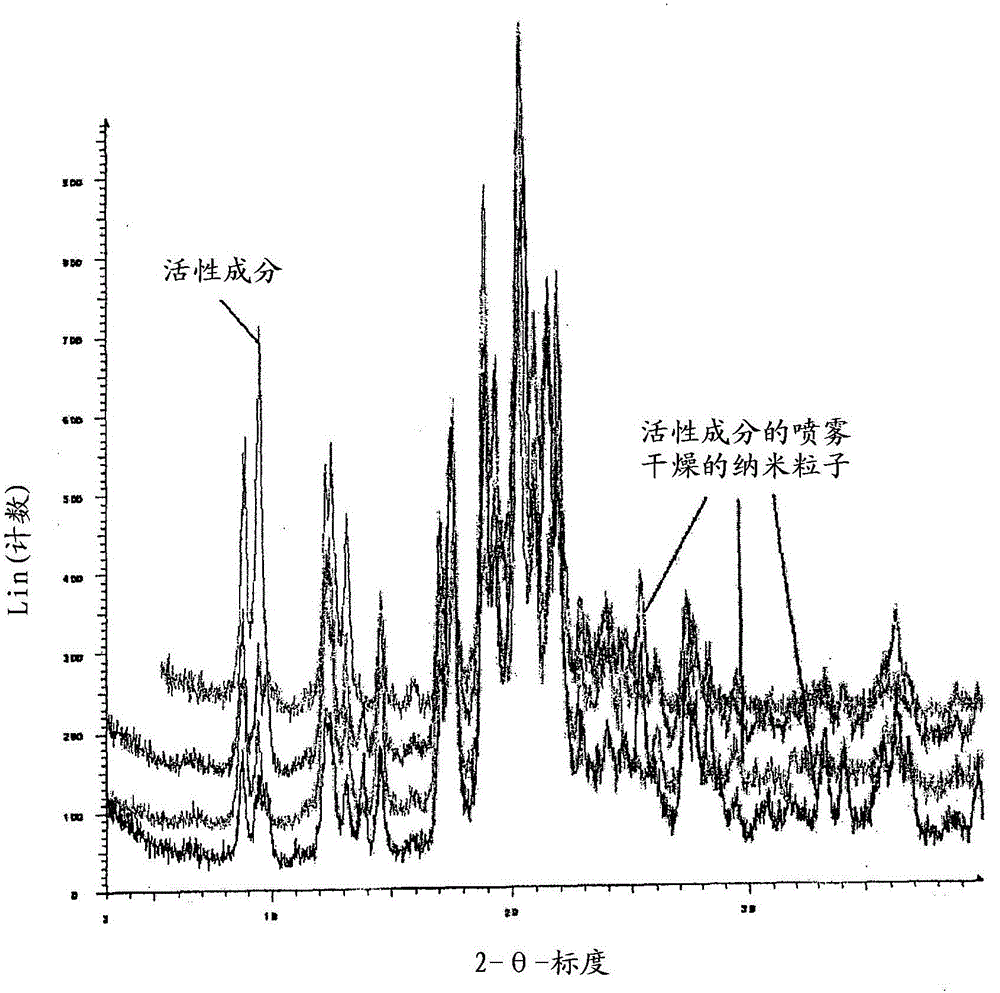
[0098] figure 1 Shown is {2-[3-cyclohexyl-3-(trans-4- X-ray ...
Embodiment 2
[0106] Nanosuspensions of {2-[3-cyclohexyl-3-(trans-4-propoxy-cyclohexyl)-ureido]-thiazol-5-ylsulfanyl}-acetic acid were prepared using the method described above, wherein The polymer stabilizer was hydroxypropyl cellulose (HPC) and the surfactant stabilizer was sodium lauryl sulfate (SLS). The resulting nanosuspension had a solids content of 10%, an average particle size of 252.2 nm, a polydispersity index of 0.171 and a zeta potential of -55.6 mV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com