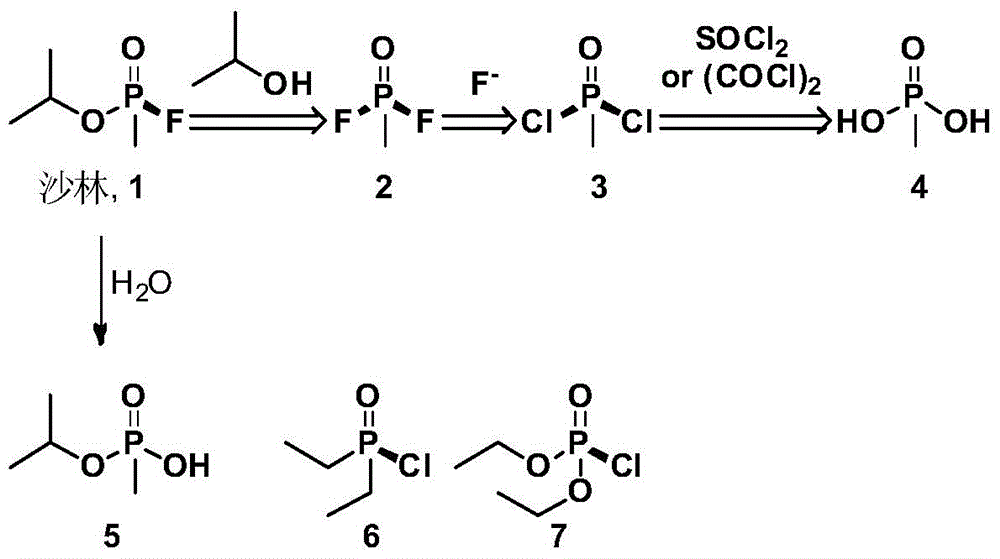
Reagent and method for detecting nerve agent and analogues thereof
A kit and solvent technology, applied in the field of nerve agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0108] The preparation of the compound of formula I of the present invention can be as shown in the following scheme 1, comprising reacting the compound of the following formula III and formula IV in the presence of a base, thereby obtaining the compound of formula I,
[0109] Process 1
[0110]
[0111] In formula III, IV and I, Z is halogen, R 1 -R 9 as defined above.
[0112] Suitable bases for the above method include, but are not limited to, sodium hydroxide, potassium hydroxide, potassium carbonate, sodium carbonate, triethylamine, diethylisopropylamine.
[0113] The reaction can also be carried out in a reaction solvent. Suitable reaction solvents include, but are not limited to, high boiling point, aprotic solvents such as dimethylformamide (DMF), dimethyl sulfoxide (DMSO), acetonitrile (CH 3 CN), 1,4-dioxane, etc.
[0114] The reaction can be carried out under heating. For example, the reaction temperature is usually 100-160°C, such as 130-150°C. The reactio...
Embodiment 1
[0138]
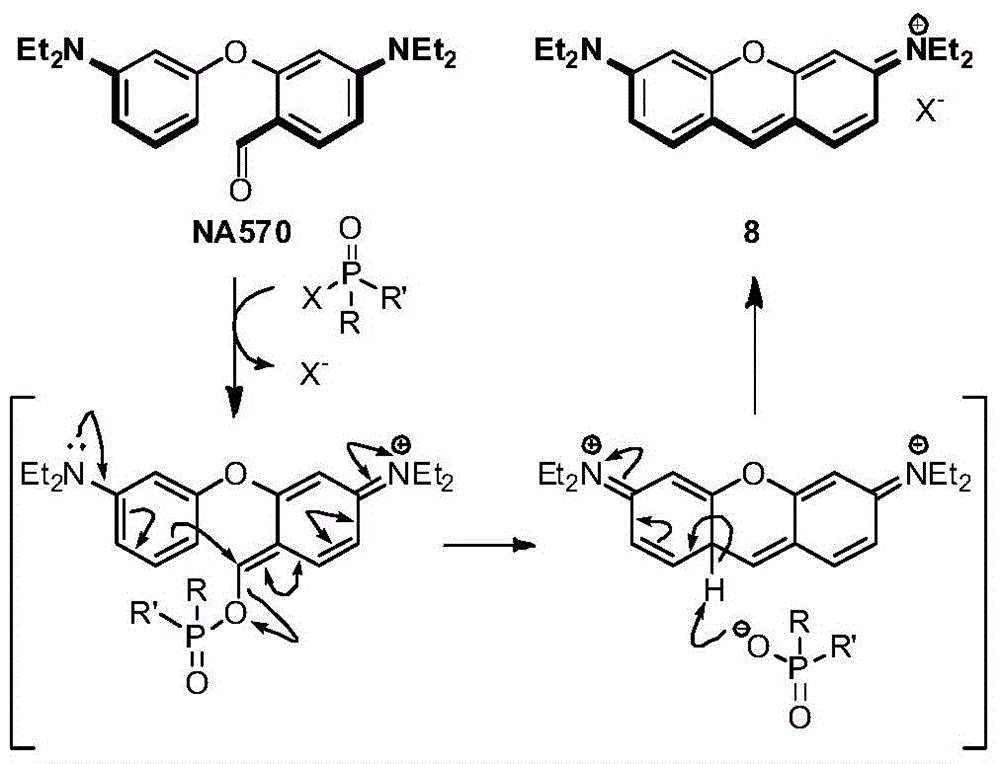
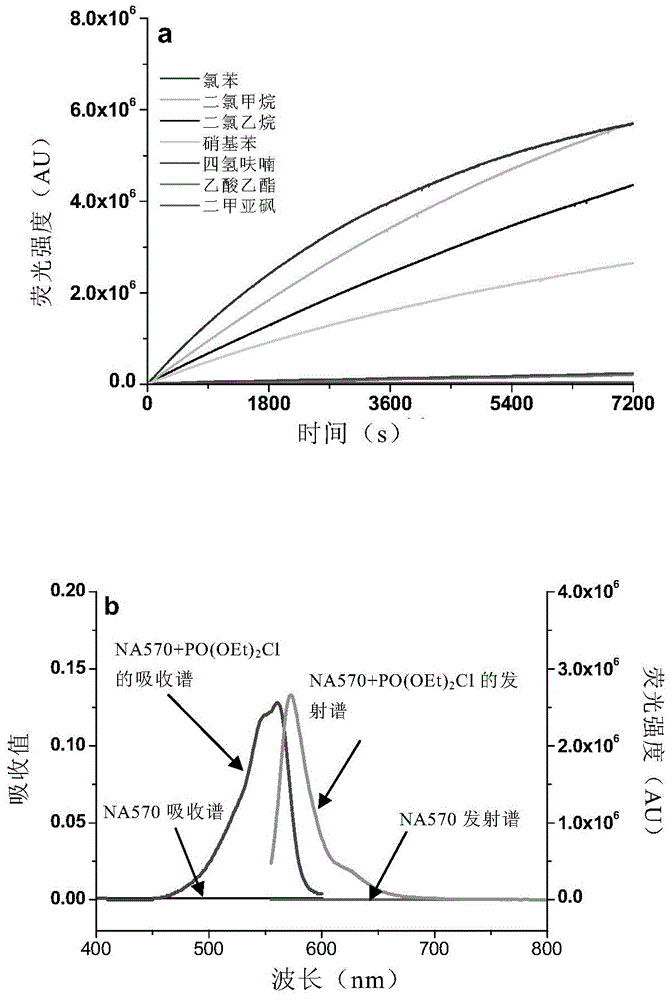
[0139] Synthesis of 4-(diethylamino)-2-(3-(diethylamino)phenoxy)benzaldehyde (NA570).
[0140] Compound 9 (1g, 6.05mmol), compound 10 (1g, 3.9mmol) and Na 2 CO 3 (620 mg, 6.05 mmol) in 40 mL of DMF was heated to 140 °C while stirring was continued for 6 hours. The mixture was then cooled to room temperature and the solids were filtered off. Removal of DMF under reduced pressure gave a viscous residue which was purified by flash chromatography using a mixture of petroleum ether and EtOAc (20:1, v / v) as NA570 (1.04 g, colorless solid) , the yield was 78%.
[0141] 1 H NMR (400MHz, CDCl 3 ):δ10.15(s,1H),7.80(d,J=8.0Hz,1H),7.13(t,J=8.0Hz,1H),6.44(d,J=8.0Hz1H),6.42(d,J =8.0Hz,1H),6.40(d,1H),6.25(dd,J=8.0Hz1H),6.07(d,J=2.4Hz,1H),3.32(m,8H),1.14(m,12H);
[0142] 13 C NMR (100MHz, CDCl 3 ): δ187.0, 162.2, 158.3, 153.6, 149.5, 130.2, 129.9, 115.9, 107.3, 106.8, 105.2, 102.499.9, 44.8, 44.4, 12.6, 12.5;
[0143] EI-HRMS(m / z):[M+H] + C 21 h 28 N 2 o 2 , the cal...
Embodiment 2
[0146]
[0147] Compound NA590 was prepared according to the method of Example 1, except that 3-hydroxydoloidine was used instead of 3-diethylaminophenol. NA590's 1 H NMR, 13 C NMR and HRMS data are shown below:
[0148] 1 H NMR (400MHz, CDCl 3 ): δ9.89(s,1H),7.46(s,1H),7.04(t,J=8.0Hz,1H),6.33(d,J=8.0Hz1H),6.32(d,J=4.0Hz,1H ), 5.97(dd, J=8Hz, 1H), 3.32(m, 6H), 3.26(t, J=8Hz, 2H), 2.78(t, J=8Hz, 2H), 2.59(t, J=8Hz, 2H), 1.99(m, 2H), 1.89(m, 2H), 1.16(t, J=8Hz, 6H);
[0149] 13 C NMR (100MHz, CDCl 3 ): δ187.71, 160.60, 154.60, 149.42, 149.10, 130.04, 126.12, 117.94, 117.35, 112.81, 105.68, 101.49, 98.89, 50.05, 49.68, 44.41, 25.39, 11.39, 20.21
[0150] HRMS[M+H] + :365.2232.
[0151] NA590's 1 H NMR and 13 C NMR spectrum as attached Figure 8-9 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com