IgG binding epitopes of main soybean allergen Gly m Bd 28K
A technology of major allergens and binding sites, applied in the fields of bioinformatics, molecular biology, and immunology, which can solve problems such as elevated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
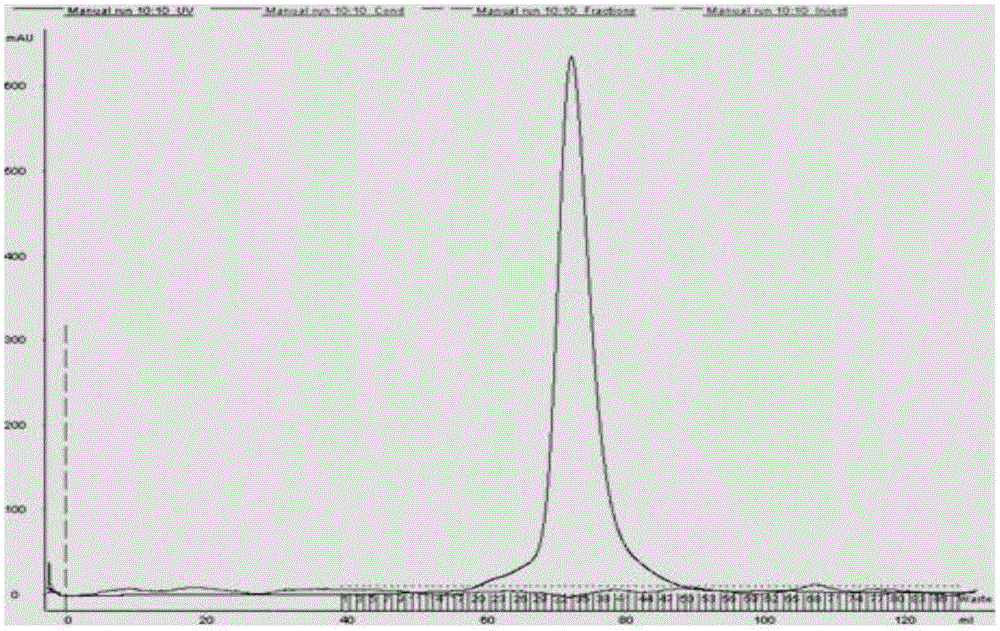
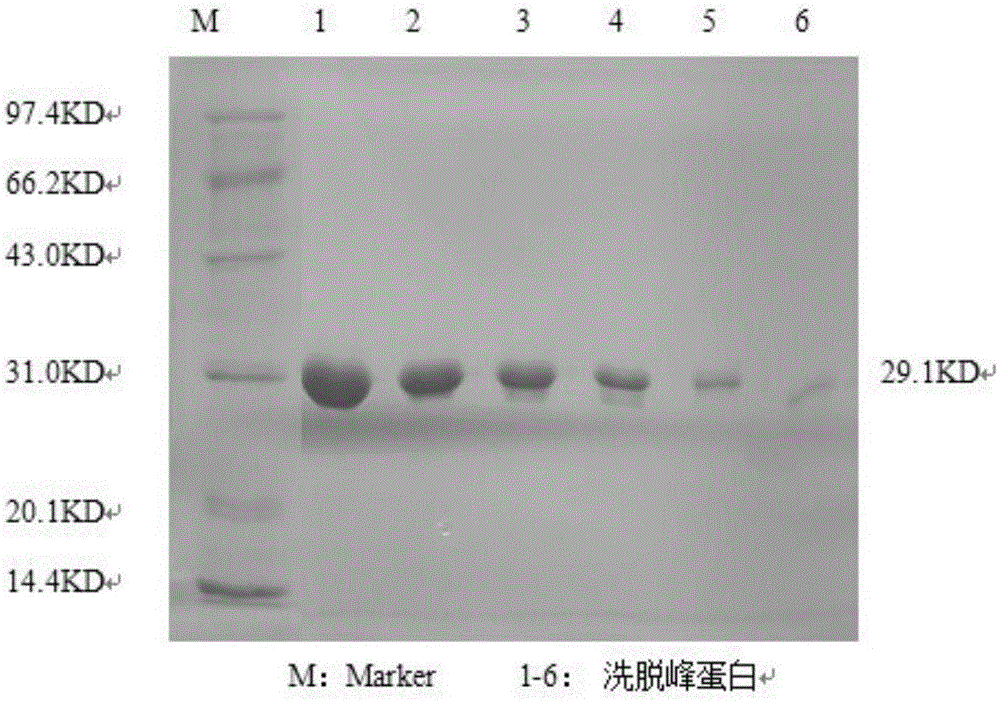
[0031] 1. Preparation of Gly m Bd 28K recombinant protein
[0032] Expression of 1 Gly m Bd 28K recombinant protein
[0033] Based on the known Gly m Bd 28K sequence in the NCBI Genbank gene bank (accession number: 21619.2), primers were designed for PCR amplification. The PCR product was detected and recovered, and connected with PMDT-19 to construct the cloning vector 28K-T. The 28K-T and pET-28a plasmids were digested with EcoRI and XhoI, respectively, to construct the recombinant plasmid pET-28K, which was transformed into Escherichia coli, and the expression of Gly m Bd 28K recombinant protein was induced by IPTG.
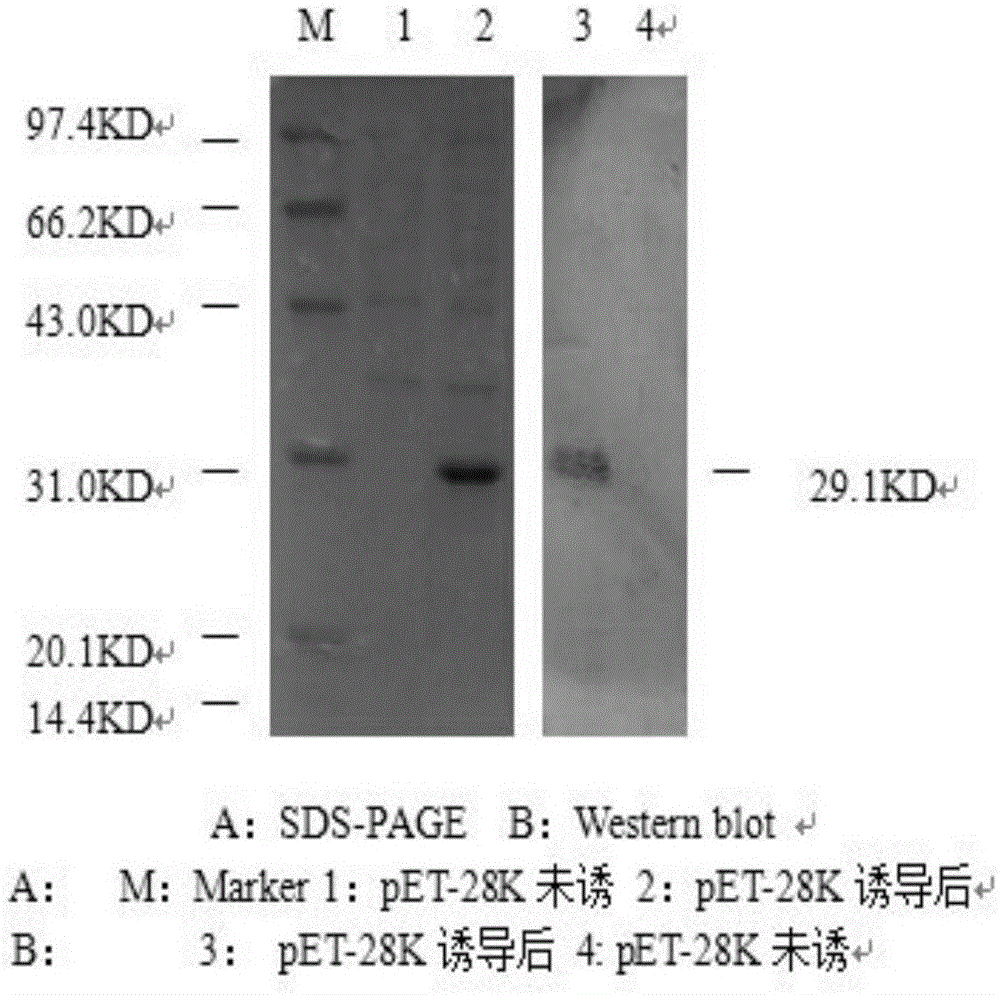
[0034] 2 Western blot detection
[0035] After IPTG-induced pET-28K whole bacteria and uninduced whole bacteria were subjected to 12% SDS-PAGE, the proteins separated by SDS-PAGE electrophoresis were charged and transferred to PVDF western blot membrane, blocked with 5% skimmed milk powder at 37°C for 1 hour, and washed with Tris The buffer was fully washed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| reaction rate constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com