Rupatadine fumarate granule and preparation method thereof
A technology of rupatadine fumarate and granules, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Convenience and other issues, to achieve the effect of small moisture residue, suitable for large-scale production applications, and low moisture content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
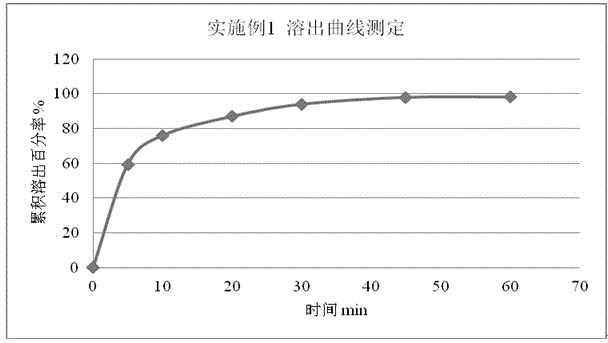
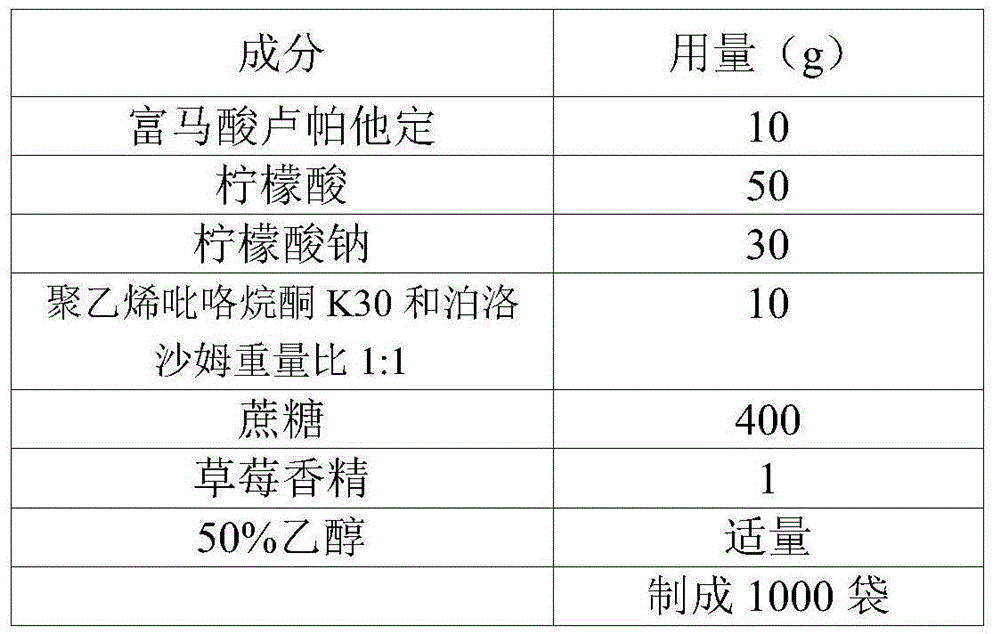
Embodiment 1
[0049] Embodiment 1 prescription
[0050]
[0051] Preparation Process:
[0052] 1) Crushing the raw material of rupatadine fumarate and passing through a 200-mesh sieve;
[0053] 2) Take sucrose, citric acid, and sodium citrate that have passed through a 80-100 mesh sieve.
[0054] 3) Put the weighed raw and auxiliary materials in a wet mixing granulator, stir and mix for about 4 minutes, add an appropriate amount of 50% ethanol solution of polyvinylpyrrolidone K30 and poloxamer in a weight ratio of 1:1, and make wet granules;
[0055] 4) Use a 24-mesh sieve to sieve the granules, spread the prepared granules on a baking tray, and send them to a hot air circulation oven for drying. The temperature is controlled below 65°C, and the moisture content is ≤3.0%;
[0056] 5) After sizing the dry granules, add strawberry essence to the granules, mix them evenly, check the content, and pack them into bags according to the dosage.
Embodiment 2
[0057] Embodiment 2 prescription
[0058]
[0059] The preparation method is as in Example 1.
Embodiment 3
[0060] Embodiment 3 prescription
[0061]
[0062] The preparation method is as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com