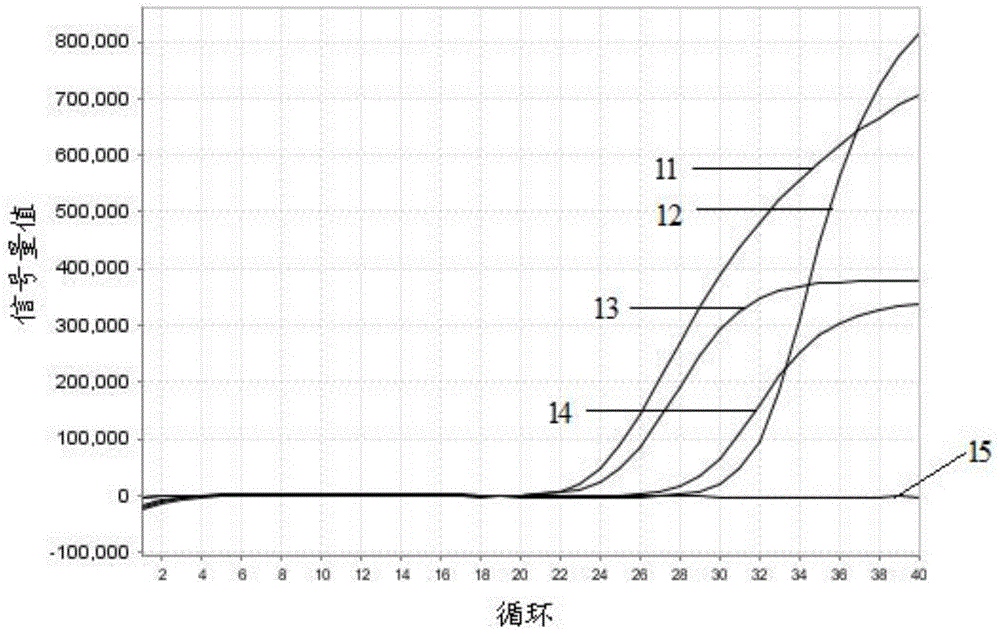
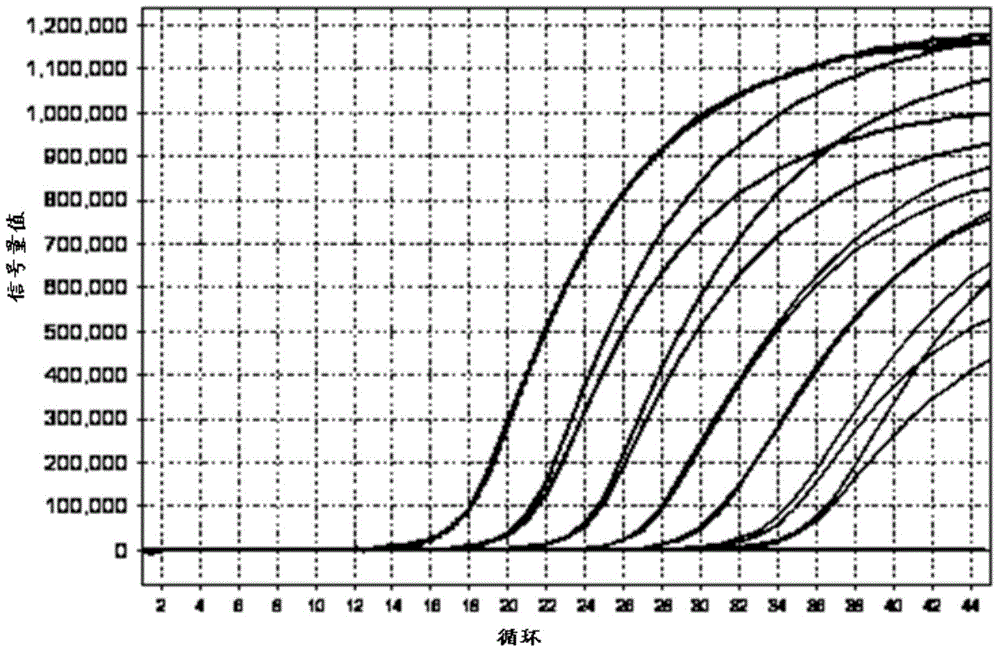
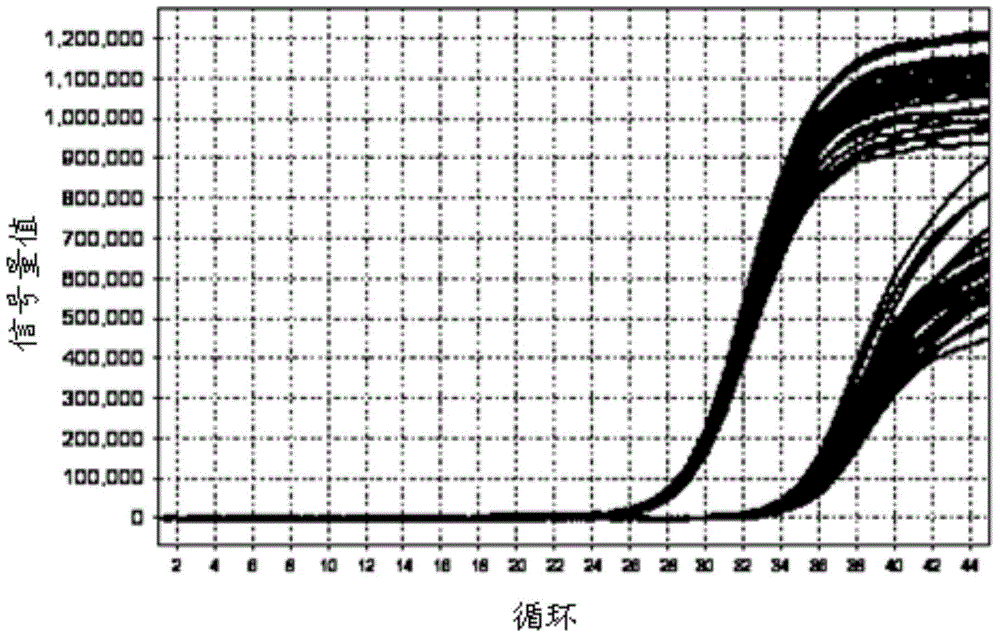
Kit for detecting mycoplasma hominis nucleic acid through PCR-fluorescence probe method and detecting method of kit
A technology of mycoplasma hominis and fluorescent probe, which is applied in the field of medical detection and human virus detection, can solve the problems of lack of negative, positive control, high detection sensitivity, and insufficient specificity, so as to reduce false positive results, accurate detection results, The direct effect of identification results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0083] In order to describe the technical content of the present invention more clearly, further description will be given below in conjunction with specific embodiments.
[0084] One, the main components of the PCR-fluorescent probe method detection Mycoplasma hominis nucleic acid kit are shown in Table 1:
[0085] Table 1
[0086]
[0087] Prepare your own sterilized saline (0.9% NaCl), absolute ethanol, and 1.5ml EP tubes, and components from different batches cannot be mixed.
[0088] The nucleic acid kit for detection of Mycoplasma hominis by PCR-fluorescence probe method comprises: nucleic acid extraction solution, MH PCR amplification solution, MH positive control and negative control.
[0089] (1) Lysis solution: 5 mol / liter (M) guanidine hydrochloride, 0.5M potassium chloride, 10 mmol / liter (mM) tris(Tris), 1mM ethylenediaminetetraacetic acid (EDTA), 0.1% Triton X-100 (volume ratio), pH 6.4 at 25°C.
[0090] Proteinase K: 0.02 grams per liter (g / L), 1mM Tris, 1m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com