Method for detecting telomerase activity
A technology for telomerase and activity, which is applied in the field of detection of telomerase activity, can solve the problems of probes being difficult to obtain and popularize, and achieve the effects of simple operation, improved sensitivity and specificity, and low detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Step 1: Preparation of Telomerase Extract
[0061] Firstly, telomerase was extracted from bladder cancer cells E-J, breast cancer cells MCF-7, cervical cancer cells HeLa and human embryonic lung fibroblasts by CHAPS method. Put the trypsin solution containing the sample cells to be tested in a centrifuge tube, centrifuge at 160g for 5min, discard the supernatant, wash the cells 3 times with PBS, centrifuge at 160g for 5min after each wash, and take the third wash Then, 10 μL of PBS suspension containing cells was counted. Add the cells to a certain volume of RNase inhibitor-treated ice-cold CHAPS lysate, the control concentration is 5000 cells / μL, put the mixture in ice for 30min, then centrifuge at 12000g for 20min at 4℃, and take the supernatant , which is the telomerase extract, which was stored at -75°C for later use. Wherein the CHAPS lysate includes 10mM Tris-HCl pH7.5, 1mM MgCl 2 , 1mM EGTA, 0.1mM phenylmethylsulfonyl fluoride (PMSF), 5mM β-mercaptoethanol, 0....
Embodiment 2
[0067] Repeat Example 1 with the same steps described above, the difference is that the samples to be tested are bladder cancer cells E-J, breast cancer cells MCF-7, and cervical cancer cells HeLa; after step 1, heat inactivation of telomerase was also carried out , that is, keeping the telomerase extract in a 94° C. water bath for 20 minutes to inactivate the telomerase; in step 2, a reaction system includes the telomerase extract equivalent to 10,000 cells.
Embodiment 3
[0069] Repeat Example 1 with the same steps described above, the difference is that step 2 is directly entered without step 1, and 2 μL of 8U / μL BstDNA polymerase, 2 μL of 0.05% trypsin, 2 μL of 1 μM thrombin, and 1 mg / mL bovine serum Albumin 2 μL, and cell lysis buffer 2 μL instead of telomerase extract.
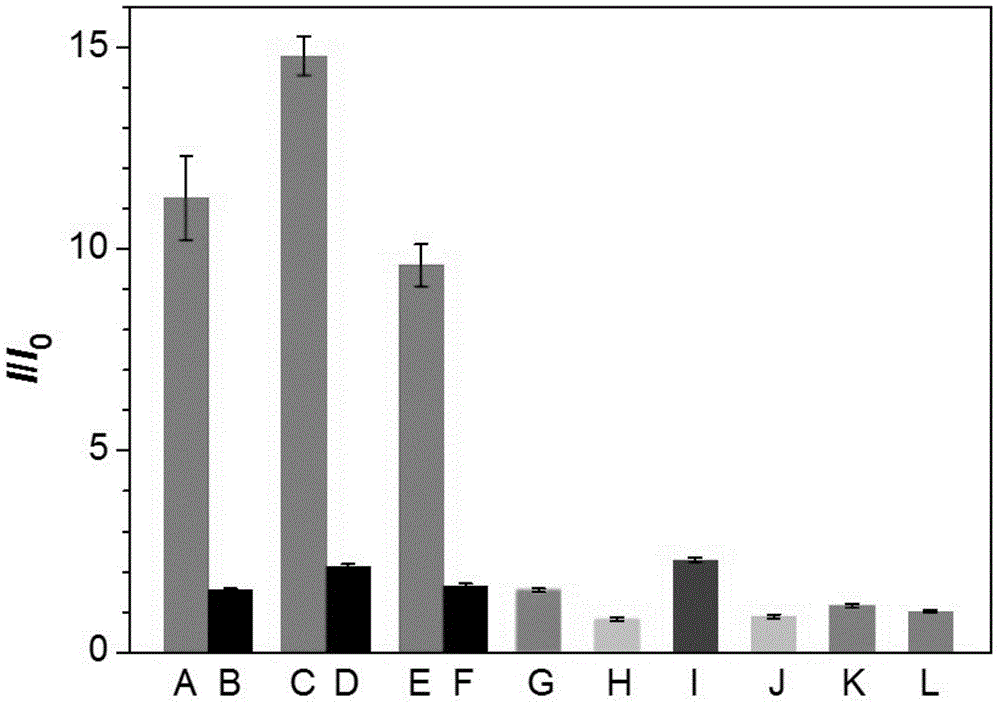
[0070] The fluorescence detection result of embodiment 1-3 is as figure 2 shown. Among them, A is the active telomerase extracted from bladder cancer cells E-J, B is the heat-inactivated telomerase extracted from bladder cancer cells E-J, C is the active telomerase extracted from breast cancer cell MCF-7, and D is Heat-inactivated telomerase extracted from breast cancer cell MCF-7, E is active telomerase extracted from cervical cancer cell HeLa, F is heat-inactivated telomerase extracted from cervical cancer cell HeLa, G is Active telomerase extracted from human embryonic lung fibroblasts, H is cell lysis buffer, I is Bst DNA polymerase, J is trypsin, K is thrombin, L is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com