Preparation method of bay-bit embedded pentabasic sulfur heterocycle and hexahydric oxygen heterocycle 3,4:9,10-perylene tetracarboxylic n-butyl acrylate
A technology for nitrating monothiocyclic perylene tetracarboxylic acid and perylene tetracarboxylic acid, which can be used in organic chemistry and other directions, can solve complex problems, and achieve the effects of expanding conjugated system, mild reaction conditions, and enhancing molecular planarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
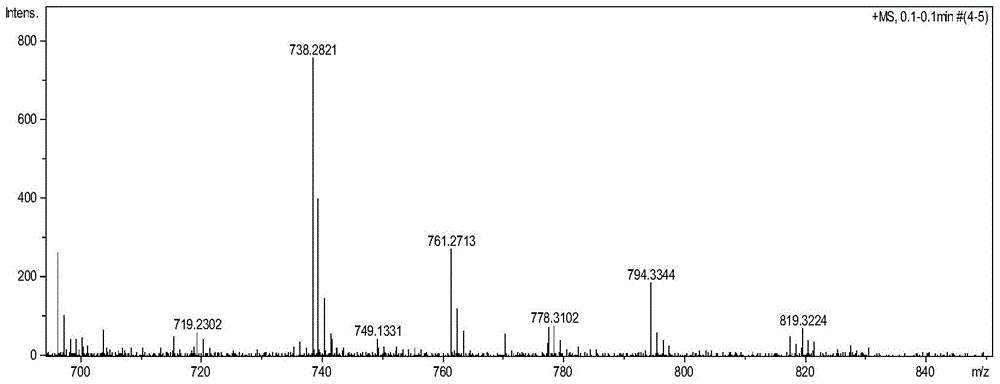
[0040] (1) Preparation of five-membered sulfur heterocyclic perylene tetracarboxylate n-butyl ester in bay position:
[0041] Add 40 mg (1.25 mmol) of sulfur powder and 8 ml of N-methylpyrrolidone (NMP) into a 25 ml round bottom flask, and stir at 90° C. for 10 minutes. Under argon protection, 50 mg (0.073 mmol) of n-butyl mononitrated perylene tetracarboxylate was added, reacted at 110°C for 6 hours, and followed the reaction by thin-layer chromatography. After the reaction, the reaction solution was poured into 100 ml of water to precipitate a precipitate, left to stand, and filtered under reduced pressure, the filter cake was washed 2 to 3 times with 10% ethanol aqueous solution, and the collected solid was vacuum-dried to obtain a crude product. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane:ethyl acetate=20:1. Obtained 35 mg (0.051 mmol, about 70% yield) of intermediate product.
[0042] (2) The preparation of mon...
Embodiment 2
[0049] (1) Preparation of five-membered sulfur heterocyclic perylene tetracarboxylate n-butyl ester in bay position:
[0050] Add 40 mg (1.25 mmol) of sulfur powder and 10 ml of N,N-dimethylformamide into a 25 ml round bottom flask, and stir at 90° C. for 10 minutes. Add 50 mg (0.073 mmol) n-butyl mononitrated perylenetetracarboxylate under nitrogen protection, react at 120° C. for 5 hours, follow the reaction by thin-layer chromatography. After the reaction, the reaction solution was poured into 100 ml of water to precipitate a precipitate, left to stand, and filtered under reduced pressure, the filter cake was washed 2 to 3 times with 10% ethanol aqueous solution, and the collected solid was vacuum-dried to obtain a crude product. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane:ethyl acetate=20:1. Obtained 35 mg (0.051 mmol, about 70% yield) of intermediate product.
[0051] (2) The preparation of mono-nitration and e...
Embodiment 3
[0057] (1) Preparation of five-membered sulfur heterocyclic perylene tetracarboxylate n-butyl ester in bay position:
[0058] Add 40 mg (1.25 mmol) of sulfur powder and 8 ml of dimethyl sulfoxide into a 25 ml round bottom flask, and stir at 90° C. for 10 minutes. Under argon protection, 50 mg (0.073 mmol) of n-butyl mononitrated perylene tetracarboxylate was added, reacted at 120°C for 4 hours, and followed the reaction by thin-layer chromatography. After the reaction, the reaction solution was poured into 100 ml of water to precipitate a precipitate, left to stand, and filtered under reduced pressure, the filter cake was washed 2 to 3 times with 10% ethanol aqueous solution, and the collected solid was vacuum-dried to obtain a crude product. The crude product was subjected to silica gel column chromatography, and the eluent was dichloromethane:ethyl acetate=20:1. Obtained 35 mg (0.051 mmol, about 70% yield) of intermediate product.
[0059] (2) The preparation of mono-nitra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com