Azoxystrobin synthesis method
A synthetic method, the technology of azoxystrobin, which is applied in the field of fungicide synthesis, can solve the problems of copper salts and tertiary amines with weak catalytic ability, decreased reaction rate, and sensitive reaction temperature, etc., and achieve simple reaction, improved safety, and high reaction rate. The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
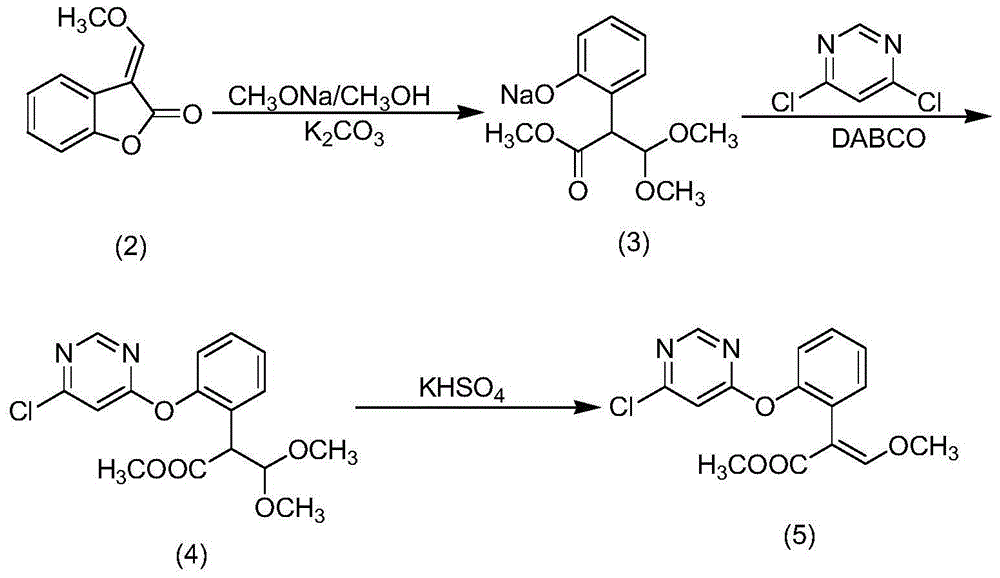
[0054] (1) Add 3-(α-methoxy)-methylenebenzofuran-2(3H)-one 179.6g (98%, 1.0mol) and 400mL methanol into a 1000mL four-neck flask, stir well and cool To -5-0°C, add 198g sodium methoxide methanol solution (30%, 1.1mol) dropwise at this temperature, continue to keep warm for 1h after the dropwise addition, sample analysis, 3-(α-methoxy)-methylene Benzofuran-2(3H)-one remained at 0.13%, and the reaction solution was neutralized by adding acetic acid (6.7g, 99%, 0.11mol) and kept at room temperature for use.
[0055] (2) Put 126g (95%, 1mol) of salicylonitrile solid, 60g (99%, 0.56mol) of sodium carbonate, and 500mL of toluene into a 2000mL reaction flask, heat and reflux for dehydration, cool to room temperature after dehydration, and then add 152g (98%, 1mol) of 4,6-dichloropyrimidine, reacted at room temperature, took a sample for 4 hours, HPLC analysis showed that the content of 4-chloro-6-(2-cyanophenoxy)pyrimidine was 96.7%, 4,6-dichloropyrimidine The content of pyrimidine ...
Embodiment 2
[0058] (1) Add 3-(α-methoxy)-methylenebenzofuran-2(3H)-one 179.6g (98%, 1.0mol) and 400mL methanol into a 1000mL four-neck flask, stir well and cool To -5-0°C, add 198g sodium methoxide methanol solution (30%, 1.1mol) dropwise at this temperature, continue to keep warm for 1h after the dropwise addition, sample analysis, 3-(α-methoxy)-methylene Benzofuran-2(3H)-one remained at 0.28%, and the reaction solution was neutralized by adding acetic acid (6.7g, 99%, 0.11mol) and kept at room temperature for use.
[0059] (2) Put 126g (95%, 1mol) of salicylonitrile solid into a 2000mL reaction flask, 60g (99%, 0.56mol) of sodium carbonate, 500mL of 4-methyl-2-pentanone, heat and reflux for dehydration, anhydrous After removal, cool to room temperature, then add 152g (98%, 1mol) of 4,6-dichloropyrimidine, react at room temperature, take samples for 4h, HPLC analysis shows that the content of 4-chloro-6-(2-cyanophenoxy)pyrimidine is 97.5 %, the content of 4,6-dichloropyrimidine is less ...
Embodiment 3
[0062] (1) Add 3-(α-methoxy)-methylenebenzofuran-2(3H)-one 179.6g (98%, 1.0mol) and 400mL methanol into a 1000mL four-neck flask, stir well and cool To -5-0°C, add 198g sodium methoxide methanol solution (30%, 1.1mol) dropwise at this temperature, continue to keep warm for 1h after the dropwise addition, sample analysis, 3-(α-methoxy)-methylene Benzofuran-2(3H)-one remained at 0.05%, and the reaction solution was neutralized by adding acetic anhydride (6.2 g, 99%, 0.06 mol) and kept at room temperature for use.
[0063] (2) Put 152g (98%, 1mol) of 4,6-dichloropyrimidine and 750mL of N,N-dimethylformamide into a 2000mL reaction flask, and add 148.5g (95%, 1mol) of sodium salicylonitrile solid in batches. 1mol), reacted at room temperature for 4 hours and took samples. HPLC analysis showed that the content of 4-chloro-6-(2-cyanophenoxy)pyrimidine was 96.5%, and the content of 4,6-dichloropyrimidine was less than 1%. The reaction solution was set aside.
[0064] (3) Add 3.5 g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com