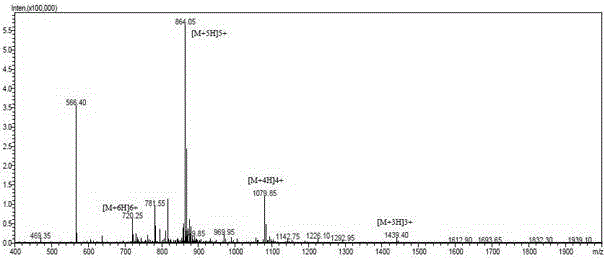
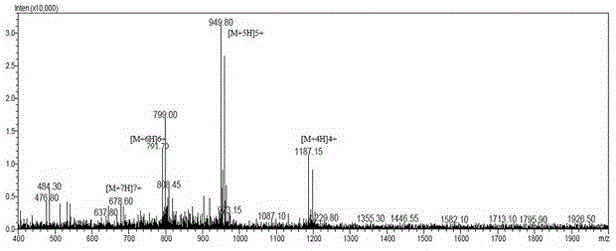
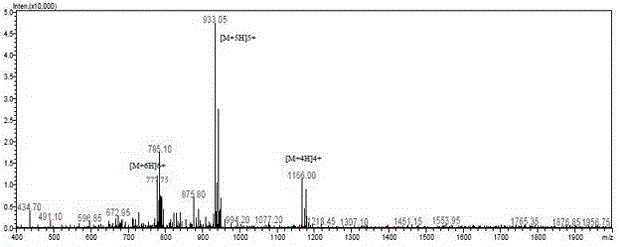
Structure-modified GLP-1 analogs and preparation method thereof
A technology of GLP-1 and analogs, which is applied in the field of preparation of exenatide derivatives and structurally modified exenatide derivatives, which can solve the problems of low toxicity and short drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1 The preparation of compound shown in formula 10
[0135]
[0136]
[0137] Under nitrogen protection, add 200 mL of pyridine and 120 g of the compound shown in formula 1 (1.0eq) into a 1000ml three-necked flask, stir to cool down to 0°C, add 151.8g of TsCl (1.0eq) in batches, stir for 1h, and then slowly raise the temperature to At room temperature, continue to stir for 3-4h. After the reaction is over, pour the reaction solution into ice dilute hydrochloric acid solution, solids are produced, add EA for extraction, wash the EA layer with dilute hydrochloric acid once, wash with saturated sodium bicarbonate, and wash with saturated brine, then anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure to obtain 119 g of the crude product, which was subjected to silica gel column chromatography (eluent: petroleum ether: ethyl acetate: acetic acid = 4:1:0.1) to obtain 55 g of the pure product of formula 2.
[0138] Add 55 g (...
Embodiment 2
[0145] Example 2 The preparation of compound shown in formula 13
[0146]
[0147] Compound 12 was prepared in a similar manner to compound 9. Add 1.0g compound 7, 15ml water, 0.7g NaHCO into 100mL three-neck flask 3 , 1.32 g of compound 12 and 15 ml of DME solution were added dropwise, stirred overnight, and the progress of the reaction was detected by TLC.
[0148] Post-reaction processing:
[0149] Add water to adjust the pH to about 3, extract with EA, wash the EA layer with dilute sodium hydroxide solution, adjust the pH to about 2 in the water layer, add EA for extraction, wash with water, wash with saturated saline, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, About 1.2 g of compound 13 was obtained.
Embodiment 3
[0150] Example 3 Preparation of compound 16
[0151]
[0152] Add 6.3g HOSU, 10g compound 14 and 60ml DMF to a 100mL three-neck flask, control the temperature below 0°C, add 11.3g DCC and stir overnight.
[0153] Post-reaction treatment: suction filtration, add water to the filtrate, extract with EA, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure to obtain compound 15.
[0154] Add 1.0g of compound 7, 15ml of water and 0.7g of NaHCO into a 100mL three-neck flask 3 , a solution of 1.5 g of compound 15 and 15 ml of DME was added dropwise, and stirred overnight.
[0155] Post-reaction processing:
[0156] Add water to adjust the pH value to about 3, extract with EA, wash the EA layer with dilute sodium hydroxide solution, adjust the pH of the water layer to about 2, add EA for extraction, wash with water, wash with saturated saline, dry over anhydrous sodium sulfate, and evaporate under reduced pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com