Efficient method for selective separation of copper from ammonia-ammonium salt solution of copper, cobalt and nickel
A technology of ammonium salt solution and solution, which is applied in the field of copper hydrometallurgy to achieve the effect of high extraction efficiency and strong selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
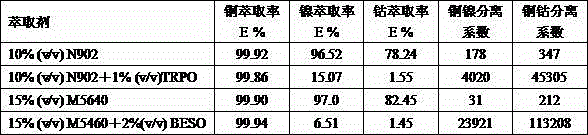
[0027] NH containing copper 3.0 g / L, nickel 3.0 g / L, cobalt 0.7 g / L 4 OH-NH 4 50 ml of Cl solution was used as the aqueous phase to be extracted, in which NH 4 OH is 4.0 mol / L, NH 4Cl is 1.0 mol / L and pH is 9.66. Prepare the organic phase, wherein the total volume of the organic phase is 50 ml, the concentration of hydroxime extractant N902 in the organic phase is 10% by volume, and adding 1% alkylphosphine oxide (code name TRPO) by volume, and the rest is diluent Sulfonated kerosene. Add the organic phase to the water phase, mechanically vibrate for 5 minutes and let it stand for stratification. After phase separation, use Hitachi Z2000 atomic absorption to measure the concentration of copper, cobalt and nickel in the raffinate water phase. Find out by subtraction. The calculated extraction rate of copper is 99.86%, the extraction rate of nickel is 15.07%, and the extraction rate of cobalt is 1.55%. The nickel separation factor is 4020, and the copper-cobalt separation ...
example 2
[0029] Prepare NH containing 3.0 g / L copper, 3.0 g / L nickel and 0.7 g / L cobalt 4 OH-NH 4 50 ml of Cl solution was used as the aqueous phase to be extracted, in which NH 4 OH is 3.75 mol / L, NH 4 Cl is 1.0 mol / L and pH is 9.45. Prepare the organic phase, wherein the total volume of the organic phase is 50 ml, the concentration of hydroxime extractant M5640 in the organic phase is 15% by volume, and 2% diisooctyl sulfoxide (code name BESO) is added to the volume ratio, and the rest Sulfonated kerosene as diluent. Add the organic phase to the water phase, mechanically vibrate for 5 minutes and let it stand for stratification. After phase separation, use Hitachi Z2000 atomic absorption to measure the concentration of copper, cobalt and nickel in the raffinate water phase. Find out by subtraction. The calculated extraction rate of copper is 99.94%, the extraction rate of nickel is 6.51%, and the extraction rate of cobalt is 1.45%. The nickel separation coefficient is 23921, an...
example 3
[0031] Prepare copper 4.5 g / L, nickel 3.6 g / L, cobalt 1.2 g / L NH 4 OH-NH 4 50ml of Cl solution is used as the aqueous phase to be extracted, in which NH 4 OH is 3.25 mol / L, NH 4 Cl is 1.0 mol / L, and the pH value is 9.35. Prepare the organic phase, wherein the total volume of the organic phase is 25ml, the concentration of the hydroxime extractant N902 in the organic phase is 10% by volume, and to add tributyl phosphate (code name TBP) with a volume ratio of 1%, the rest is diluent sulfur kerosene. Add the organic phase to the water phase, mechanically vibrate for 5 minutes and let it stand for stratification. After phase separation, use Hitachi Z2000 atomic absorption to measure the concentration of copper, cobalt and nickel in the raffinate water phase. Find out by subtraction. The calculated extraction rate of copper is 92.86%, the extraction rate of nickel is 10.06%, and the extraction rate of cobalt is 4.03%. The loaded organic phase is back-extracted cobalt with the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com