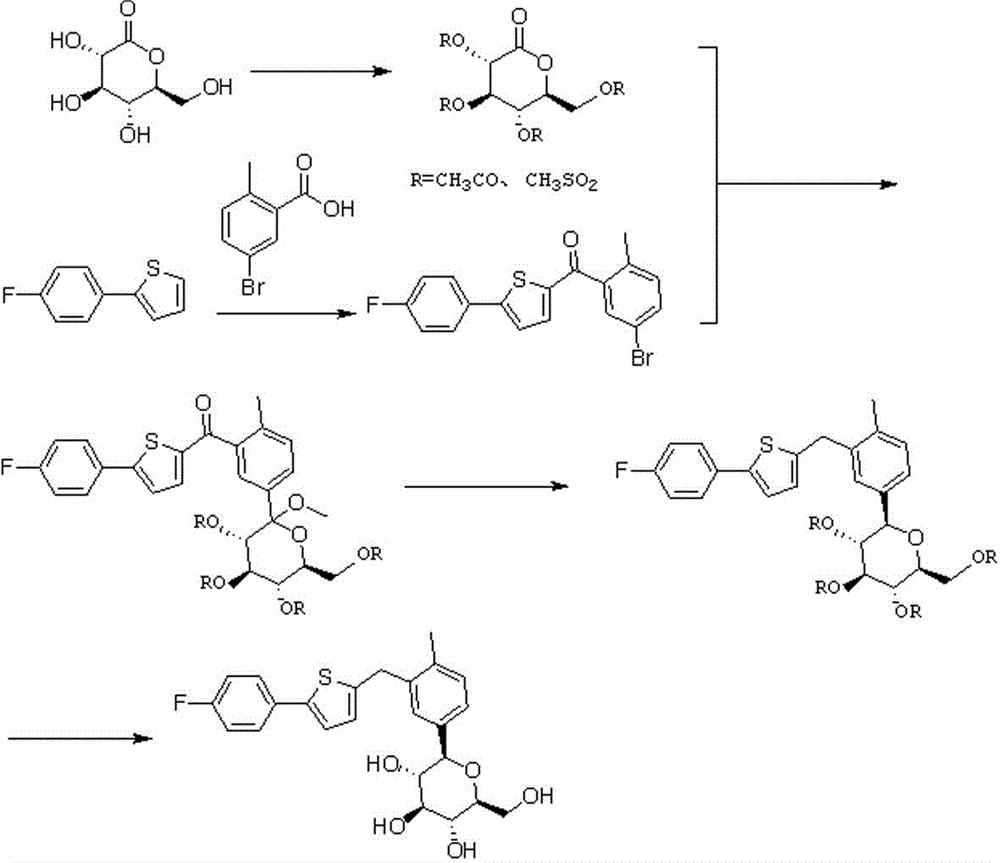
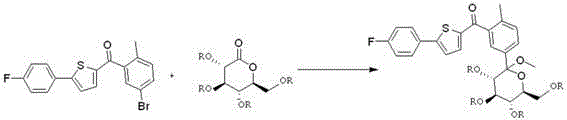
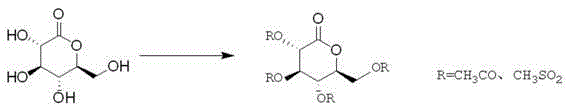
Preparation method for novel SGLT2 inhibitor medicine
An inhibitor and drug technology, applied in organic chemistry and other directions, can solve the problems of long synthetic process route, difficult solidification and purification treatment of intermediates, complicated preparation process, etc., and achieves easy quality control, low production cost and high reaction yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1). Preparation of gluconolactone intermediate
[0035] Add 500mL of toluene to the reaction flask, start stirring, add 50g of D-gluconolactone and 5g of piperidine, cool down to -5~0℃, add 30mL of N-methylpyrrolidone, control the temperature at -5~0℃, then drop Add 33.5g of acetyl chloride, and control the temperature at -5~0°C; after dropping, raise the temperature to 20-25°C, and stir overnight. After the reaction, cool down to -5~0°C, add 200mL saturated sodium bicarbonate solution, stir, let stand to separate the liquid, extract the water layer once with 300mL toluene, combine the organic phases, wash with 500mL water, and then use 300mL saturated chlorinated Wash with aqueous sodium solution, dry over anhydrous sodium sulfate, filter with suction, and concentrate the organic phase under reduced pressure to obtain 80.2 g of an oily product. The yield is 93.5%.
[0036] (2). Preparation of 2-(5-bromo-2-methylbenzoyl)-5-(4-fluorophenyl)thiophene
[0037] ...
Embodiment 2
[0045] (1). Preparation of gluconolactone intermediate
[0046] Add 700mL of dichloromethane into the reaction bottle, start stirring, add 50g of D-gluconolactone and 10g of triethylamine, cool down to -5~0°C, add 20mL of N-methylpyrrolidone, and control the temperature at -5~0°C , and then dropwise added 38.9g of methanesulfonyl chloride, temperature controlled -5~0°C; after dropping, the temperature was raised to 20-25°C, and stirred overnight. After the reaction, cool down to -5~0°C, add 300mL saturated sodium bicarbonate solution, stir, let stand for liquid separation, extract the water layer once with 500mL dichloromethane, combine the organic phases, wash with 500mL water, and then wash with 500mL saturated Wash with aqueous sodium chloride, dry over anhydrous sodium sulfate, filter with suction, and concentrate the organic phase under reduced pressure to obtain 81.5 g of an oily product. Yield 95.2%.
[0047] (2). Preparation of 2-(5-bromo-2-methylbenzoyl)-5-(...
Embodiment 3
[0056] (1). Preparation of gluconolactone intermediate
[0057] Add 500mL ether into the reaction flask, start stirring, add 40g D-gluconolactone and 6gDMAP, cool down to -5~0℃, add 10mL N-methylpyrrolidone, control the temperature at -5~0℃, and then add dropwise 38.9 g acetyl chloride, temperature control -5~0°C; after dropping, raise the temperature to 20-25°C, and stir overnight. After the reaction, cool down to -5~0°C, add 200mL saturated sodium bicarbonate solution, stir, let stand for liquid separation, extract the water layer once with 300mL ether, combine the organic phases, wash with 500mL water, and then use 500mL saturated chlorinated Wash with aqueous sodium solution, dry over anhydrous sodium sulfate, filter with suction, and concentrate the organic phase under reduced pressure to obtain 63.6 g of an oily product. Yield 95.2%.
[0058] (2). Preparation of 2-(5-bromo-2-methylbenzoyl)-5-(4-fluorophenyl)thiophene
[0059] Add 30g of 5-bromo-2-methylben...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com