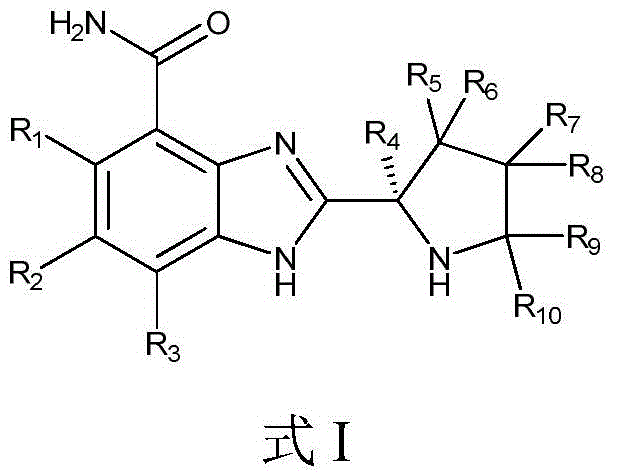
A compound for treating tumors and its application
A compound and application technology, applied in antineoplastic drugs, medical preparations containing active ingredients, organic chemistry, etc., can solve the problems of lack of exploration in the research of Veliparib analogues, achieve excellent pharmacokinetic properties, good inhibitory effect, The effect of high drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1 Preparation method one of the compound of the present invention
[0065]
[0066] Deuterated or substituted 2,3-diaminobenzamide or (10mmol) and deuterated or substituted R-1-tert-butoxycarbonyl-2-methylproline.(10mmol) were dissolved in 50mL of dichloro Methane, dicyclohexylcarbodiimide (12mmol), hydroxybenzotriazole (12mmol), triethylamine (13mmol) were added. After stirring at room temperature for 12 hours, the precipitate produced by the reaction was filtered off. The mother liquor was washed with 5% dilute hydrochloric acid, the organic phase was separated and concentrated under reduced pressure. The crude product was dissolved in 10 mL of acetic acid, heated to 70°C, and the reaction was monitored by TLC, then concentrated under reduced pressure. Dissolve the crude product in 10 mL of tetrahydrofuran, add 6M hydrochloric acid, and stir at room temperature until the reaction is complete. Add ethyl acetate with 10% Na 2 CO 3 Neutralized, the org...
Embodiment 2
[0072] Embodiment 2 is used for the preparation of the intermediate of compound of the present invention
[0073] 1. Synthesis of (R)-1-(benzyloxycarbonyl)-2-methylpyrrolidine-2-carboxylic acid (compound 2)
[0074]
[0075] Add (R)-2-methyl-2-pyrrolidine carboxylic acid hydrochloride (compound 1) (4g, 24mmol), sodium carbonate (10.2g, 96mmol), tetrahydrofuran (40mL) and water ( 40mL), benzyl chloroformate (CbzCl) (6.2g, 36mmol) was added under stirring, the mixture was separated at room temperature overnight, separated, extracted with ethyl acetate (3×30mL), the organic layer was dried over anhydrous sodium sulfate, and the solvent was concentrated , after purification with a silica gel column, white solid compound 2 (3.5 g, yield 56%) was obtained, mass spectrum: 264.1 (M+H + ).
[0076] 2, Synthesis of 2-amino-3-nitrobenzamide (compound 4)
[0077]
[0078] Add 2-amino-3-nitrobenzoic acid (compound 3) (9g, 50mmol) and tetrahydrofuran (70mL) into a 500mL single-neck...
Embodiment 3
[0085] Example 3 Synthesis of 2-[(2R)-2-methyl-5,5-dideutero-2-pyrrolidinyl]-1H-benzimidazole-4-carboxamide (compound D0002)
[0086]
[0087] 1. Synthesis of (2R)-2-methyl-2-pyrrolidine-methyl formate (compound 11)
[0088]
[0089] Add (2R)-2-methyl-2-tetrahydropyrrole carboxylate hydrochloride (compound 1) (5g, 30mmol) and methanol (50mL) to a 100mL single-necked bottle, and add dichlorohydrin dropwise under an ice bath. Sulfone (5.4g, 45mmol), return to room temperature overnight after the dropwise addition, spin out the solvent, dissolve the residue in dichloromethane (50mL), wash with a small amount of aqueous sodium bicarbonate until neutral, separate liquid, organic The layer was dried over anhydrous sodium sulfate, and the solvent was concentrated to obtain compound 11 (4.1 g, yield 95%). Mass spectrum: 144.2 (M+H + ).
[0090] 2. Synthesis of (2R)-1-tert-butoxycarbonyl-2-methylpyrrolidine-2-carboxylic acid methyl ester (compound 12)
[0091]
[0092] Add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com