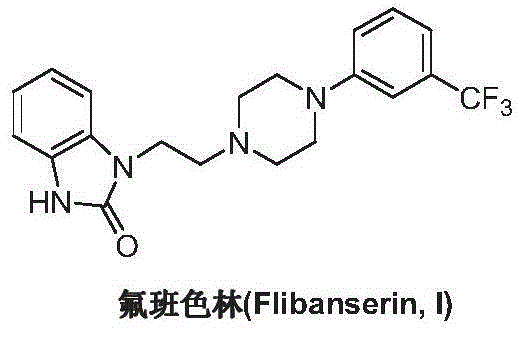
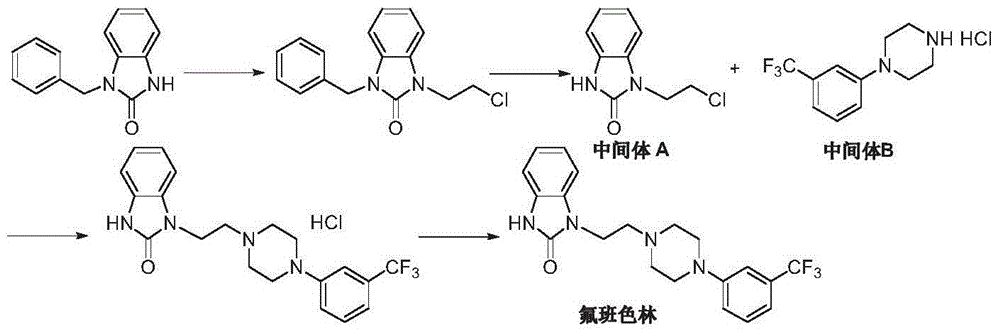
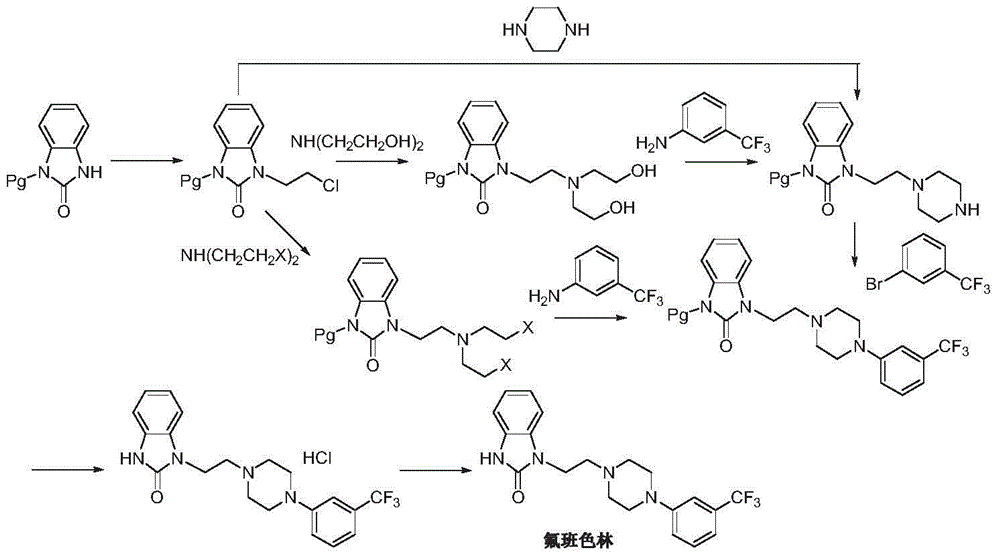
Preparation method of flibanserin
A technology of flibanserin and trifluoromethyl phenyl is applied in the field of organic synthesis route design and the preparation of raw materials and intermediates, and can solve the problems of difficulty in obtaining raw materials of the synthesis route, high control and cost, and many reaction steps. , to achieve the effect of fast and convenient preparation process, high product yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add m-aminobenzotrifluoride (8.5g, 50mmol), tris(2-chloroethyl)amine (12.2g, 60mmol), potassium carbonate (6.9g, 50mmol) and 150mL of n-butanol in the reaction flask, and heat up to 115- 120° C., stirred for 24 hours, and TLC detected that the reaction was complete. The reaction solution was concentrated, dissolved in dichloromethane, washed twice with water, the organic phase was dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. Obtained 6.7 g of yellow-green oil N-(3-trifluoromethylphenyl)-N'-(2-chloroethyl)-piperazine (II), yield 45.9%, EI-MS m / z: 293 [M+H] + .
Embodiment 2
[0036] After stirring and dissolving m-aminobenzobenzotrifluoride (8.5g, 50mmol), tris(2-bromoethyl)amine (33.4g, 100mmol) and ether 50mL, pour it into a solid phase reactor equipped with 100g of basic alumina , after stirring evenly, remove the solvent by bubbling with nitrogen gas, seal it, raise the temperature to 145-150°C, and stir for 1-2 hours to react. After cooling, the resulting mixture was washed successively with 200 mL of a 5% sodium methoxide / methanol solution and 200 mL of dichloromethane. The solutions were combined and concentrated to obtain 13.2 g of light brown viscous liquid N-(3-trifluoromethylphenyl)-N'-(2-bromoethyl)-piperazine (II), yield 78.6%, EI -MS m / z: 337[M+H] + .
Embodiment 3
[0038] Add N-(3-trifluoromethylphenyl)-N'-(2-bromoethyl)-piperazine (II) (6.7g, 20mmol), o-nitroaniline (2.8g, 20mmol) into the reaction flask ), cuprous iodide (0.38g, 2mmol), diisopropylethylamine (0.52g, 4mmol) and N,N-dimethylformamide 50mL, heated to 100-110°C under stirring, and reacted for 5 hours, TLC detects that the reaction is complete. Cool down to 50-60°C, filter, and wash the filter cake with ethyl acetate. The filtrate was washed with brine and water, concentrated, and recrystallized from ethyl acetate and n-hexane (1:1) to obtain a yellow solid N-(3-trifluoromethylphenyl)-N'-[2-(N-(2 -Nitrophenyl)amino)ethyl]-piperazine (III) 6.4 g, yield 81.2%. EI-MS m / z: 395[M+H] + , δ3.04-3.87 (m, 8H), 3.90-4.06 (m, 4H), 5.42 (brs, 1H), 6.73-7.78 (m, 8H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com