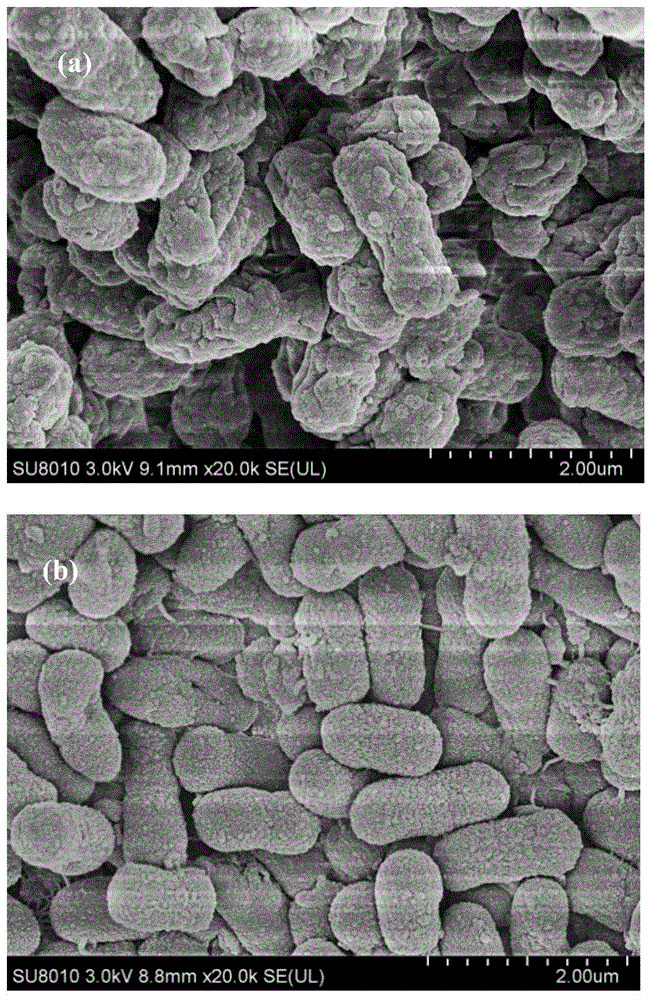
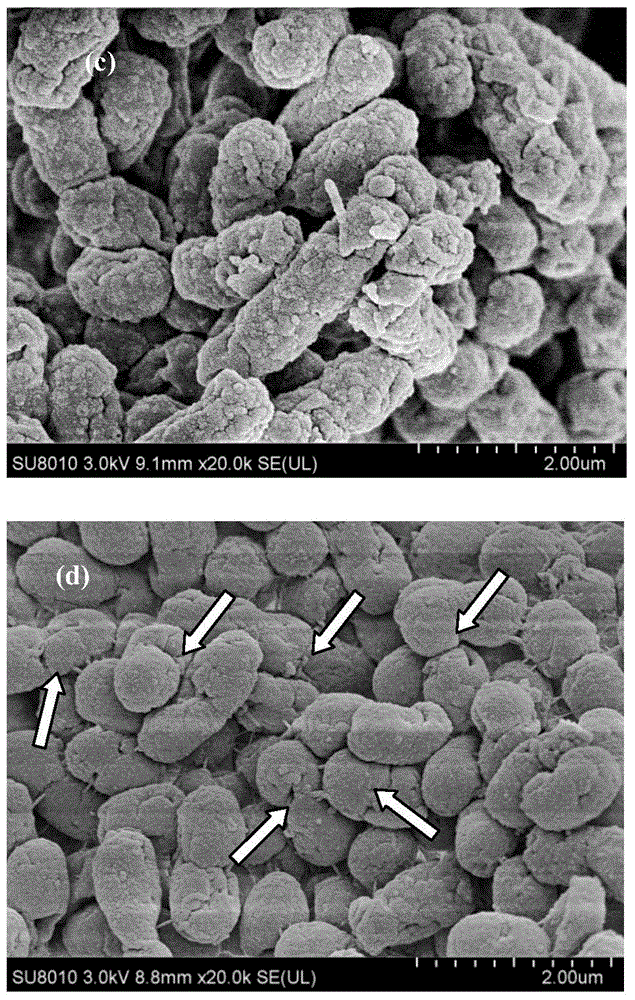
Immobilization method for cells containing nitrilase
A nitrilase and cell technology, applied in the field of immobilization of nitrilase-containing cells, can solve the problems of low reaction batch, low substrate solubility, high toxicity of bisnitrile substrates, etc., and achieves good reusability and operation. Simple, low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Construction of recombinant Escherichia coli E.coli BL21(DE3) / pET28b(+)-F168V and preparation and performance determination of wet cells
[0035] The recombinant Escherichia coli E.coli BL21(DE3) / pET28b(+)-F168V constructed in this laboratory is used as the production strain, and the method is as follows:
[0036] (1) Construction of recombinant bacteria: the Acidovorax facilis ZJB09122 nitrilase mutant gene F168V (nucleotide sequence shown in SEQ ID NO.1, has been published in Xin-Hong Zhang et al / Process Biochemistry49 (2014) 2141-2148 ) was ligated with PGEM-T vector and then introduced into E.coli JM109, F168V / PGEM-T and plasmid pET28b(+)-Nit were double digested, ligated overnight with ligase, and the ligation product pET28b(+)- F168V was introduced into host E.coli BL21(DE3) to obtain recombinant Escherichia coli E.coli BL21(DE3) / pET28b(+)-F168V.
[0037] The PGEM-T carrier connection conditions are as follows: 10 μL of the connection system is added to...
Embodiment 2
[0050] (1) Take the wet thallus obtained by the method in Example 1, mix them according to the wet weight of the thallus: phosphate buffer (pH=7.0, 100mM)=1:12 (m / v), then take 8.3g of wet thallus and add Prepare 100mL bacterial suspension in 100ml phosphate buffered saline.
[0051] (2) Add 0.4g diatomite to the 100mL bacterial suspension in step (1), mix well on the magnetic stirrer, then mix the polyethyleneimine aqueous solution with 3ml concentration of 5% (v / v) and the magnetic stirrer fully Mix under stirring for 0.5 h.
[0052] (3) Add 1 ml of 25% (v / v) glutaraldehyde aqueous solution to the solution in step (2), discard the supernatant after cross-linking at 25° C. for 0.5 h, and vacuum filter to obtain immobilized cells.
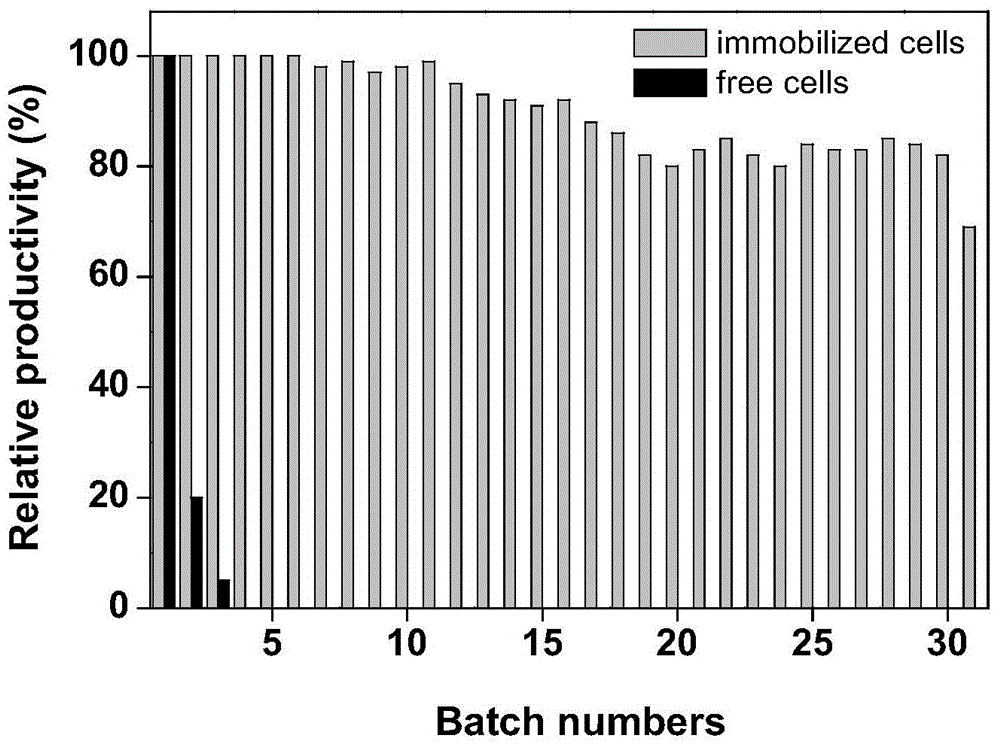
[0053] (4) Take 0.625 g of the immobilized cells prepared in step (3) in 10 ml of pH 7.0 phosphate buffer, add 200 mM substrate 1-cyanocyclohexyl acetonitrile, react at 40°C for 10 min, take samples, and detect the product 1-cyano by HPLC Accordi...
Embodiment 3
[0058] (1) Take the wet thalline prepared by the method of Example 1, mix according to the wet weight of the thallus: phosphate buffer (pH=7.0, 100mM)=1:10 (m / v) to obtain a bacterial suspension, that is, take 10g of wet thalline The bacteria were added to 100mL phosphate buffer to make 100mL bacterial suspension.
[0059] (2) in step (1) 100mL bacterium suspension, add the diatomite of 0.8g, mix on the magnetic stirrer, be the polyethyleneimine aqueous solution mixing of 5% (v / v) with 5ml concentration again, in magnetic force Mix for 1 h under vigorous agitation with a stirrer.
[0060] (3) Add 1 ml of 25% (v / v) glutaraldehyde aqueous solution to the solution in step (2), discard the supernatant after cross-linking at 15° C. for 0.5 h, and vacuum filter to obtain immobilized cells.
[0061] (4) Immobilized cell enzyme activity, enzyme activity recovery rate, half-life and continuous operation batch yield test method at 500 mM substrate concentration are the same as in Examp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com