Antitumor medicine
An anti-tumor drug and anti-tumor technology, applied in the field of anti-tumor drugs, can solve problems such as physical and mental health hazards of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 mephenamine
[0030] 2kg of Veratrum collected in Sichuan Province was ground into powder, and then extracted with 95% ethanol (20L). The extract was concentrated in vacuo to obtain 350 g of a concentrate, which was then dissolved in ethyl acetate. The organic phase was washed with purified water, and the aqueous phase was discarded. The organic phase was dried over anhydrous sodium sulfate and evaporated to dryness in vacuo, yielding 80 g of concentrate. The concentrate was loaded into a silica gel chromatography column and eluted with CHCl3-MeOH (20:1). Through this process, 200 mg of mephenamine can be obtained.
[0031] Example 2 In Vitro Test: Experimental Method for Detecting Mefenamide Inhibiting Hedgehog Signaling Pathway
[0032] 2.1 Cell culture
[0033] Gli reporter gene of Hedgehog signaling pathway-NIH3T3 cells were cultured in DMEM medium containing 10% calf serum 500 μg / ml G-418 sulfate 1% penicillin-streptomycin.
[...
Embodiment 3
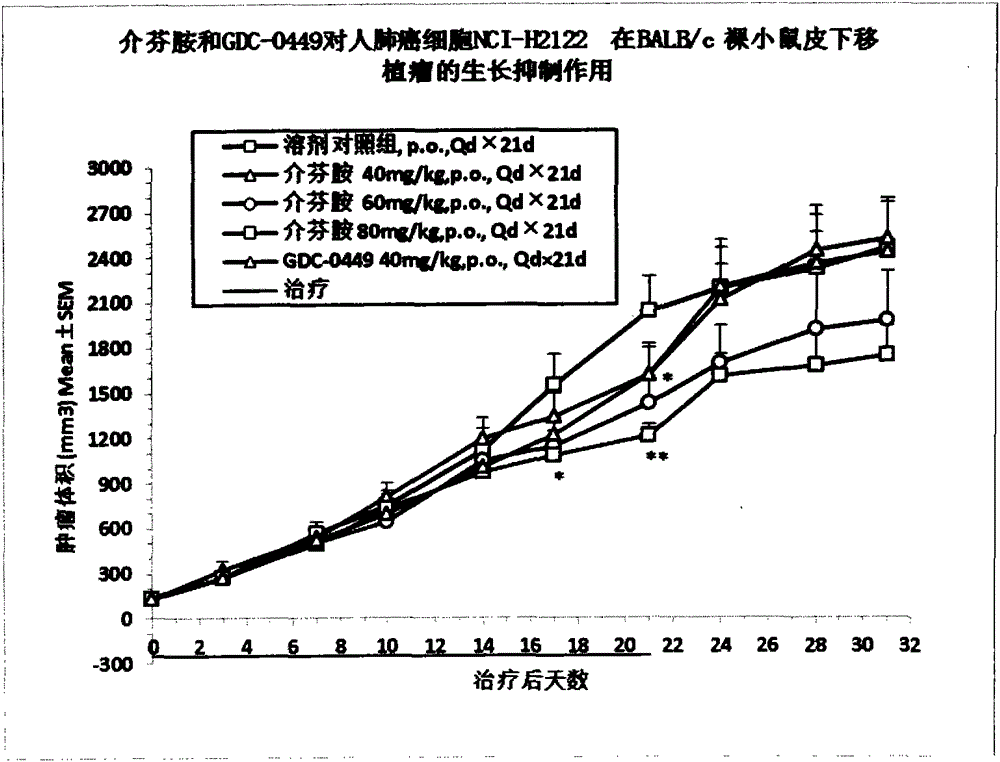
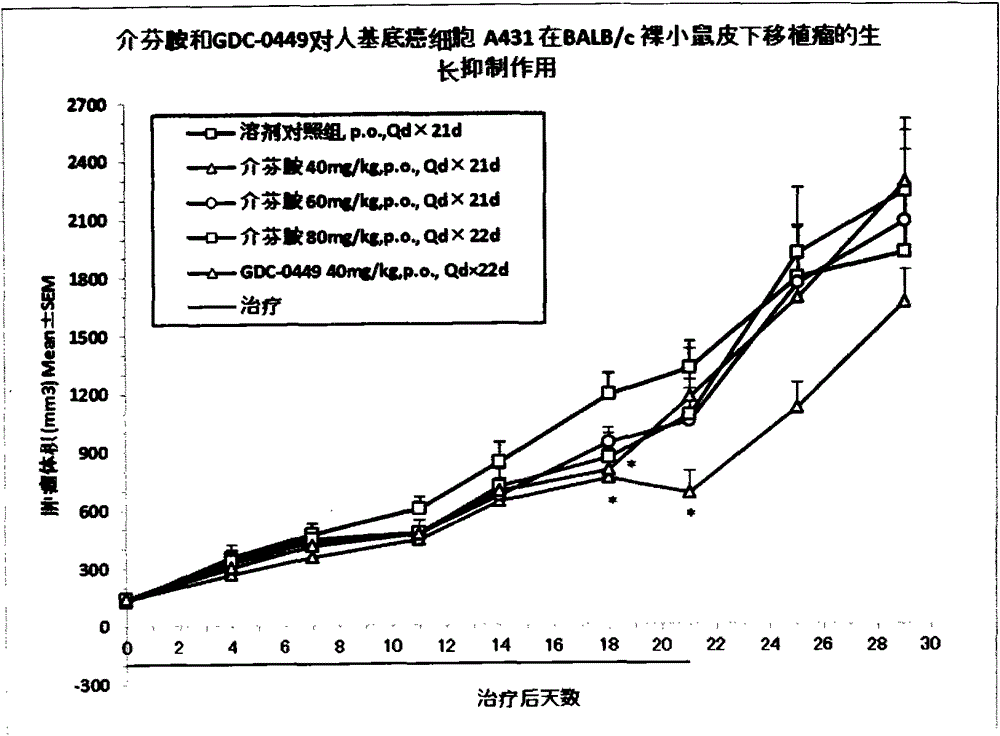
[0040] Example 3 Pharmacodynamics and drug safety evaluation of mefenamide on tumor cells NCI-H2122 and A431 in BALB / c nude mouse subcutaneous tumor model
[0041] BALB / c nude mice were subcutaneously inoculated with NCI-H2122 and A431 tumor cells to establish xenograft tumor models, and the efficacy and safety of mefenamide and GDC-0449 in these tumor models were evaluated.
[0042] 1. Cell culture
[0043] NCI-H2122 tumor cells were cultured in RMPI1640 medium containing 10% fetal bovine serum, and A431 tumor cells were cultured in DMEM medium containing 10% fetal bovine serum. All cells were digested with EDTA trypsin as usual, weekly Subcultured twice, placed in a 37°C, 5% CO2 incubator to continue culturing.
[0044] 2. Experimental animals
[0045] BALB / c nude mice, female, 6-8 weeks old, weighing about 18-22 g, 110 in number, were purchased from Shanghai Slack Experimental Animal Co., Ltd. All the mice were kept in the IVC constant temperature and constant pressure s...
Embodiment 4
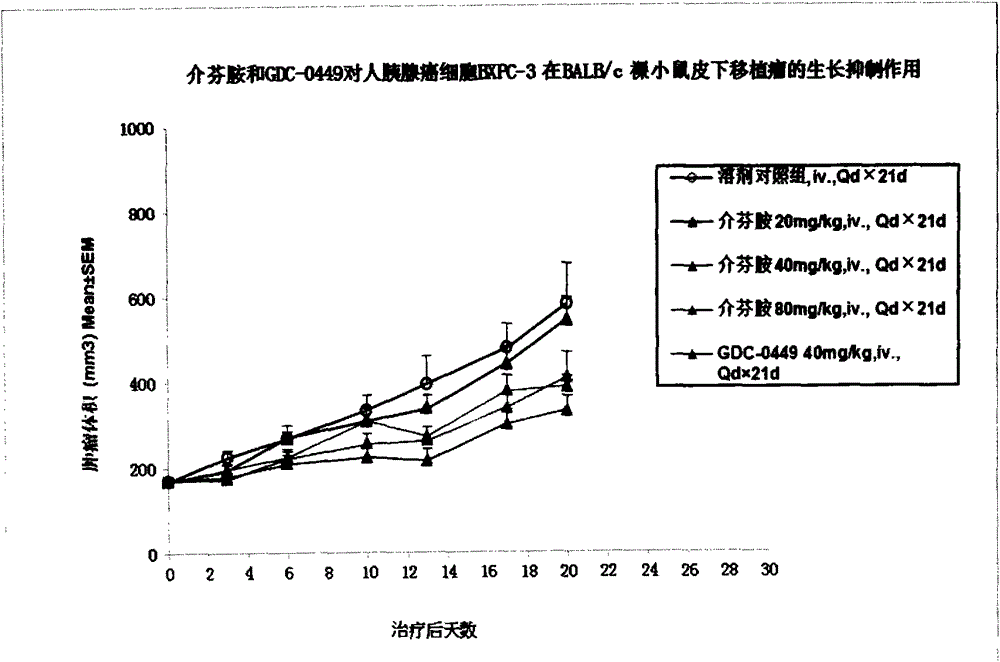
[0073] Example 4 Pharmacodynamics and drug safety evaluation of mefenamide on tumor cell BXPC-3 nude mouse subcutaneous tumor model
[0074] Xenograft tumor models were established by inoculating BXPC-3 tumor cells subcutaneously in BALB / c nude mice, and the pharmacodynamics and safety of mefenamide and GDC-0449 in these tumor models were evaluated.
[0075] cell culture
[0076] BXPC-3 tumor cells were cultured in RMPI1640 medium containing 10% fetal bovine serum. All cells were digested with trypsin containing EDTA according to routine, passed twice a week, and placed in a 37°C, 5% CO2 incubator to continue culturing .
[0077] experimental animals
[0078] BALB / c nude mice, female, 6-8 weeks old, weighing about 18-22 g, 40 in number, were purchased from Shanghai Slack Experimental Animal Co., Ltd.
[0079] Inoculate cells
[0080] Collect the logarithmic growth phase cells, adjust the concentration of BXPC-3 cells with PBS and Matrigel to (5×10 7 / ml) 0.1ml of the cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com