Method for detecting hydrogen peroxide and nitrite by electrochemical sensor based on dual-metal porphyrin coordination polymer
A technology of coordination polymer and bimetalloporphyrin is applied in the field of electrochemical detection, which can solve the problems of few reports of bifunctional electrochemical sensors, and achieve the effects of good electrocatalytic performance, fast detection speed and improved performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
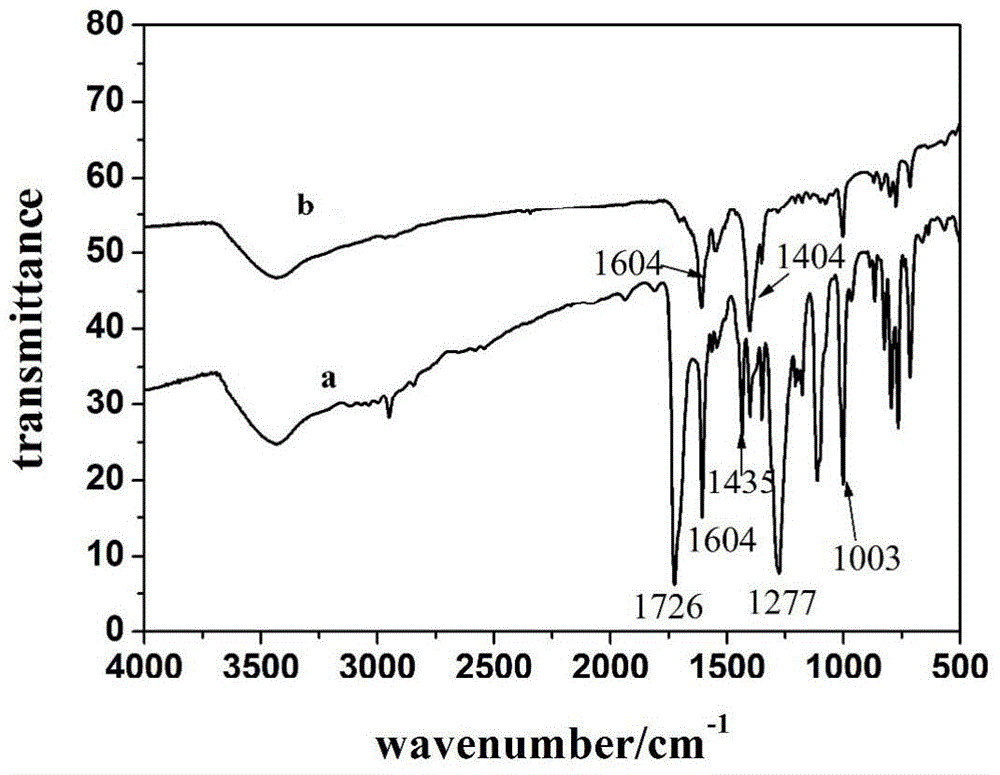
[0074] Embodiment 1 double metal coordination polymer [Cu 2 (Co-TCPP)(H 2 O)2 ]·0.5DMF·5H 2 Preparation of O(CoTCPP-Cu)
[0075] Weigh CoTCPP 3mg (0.01mmol), add DMF 3mL to dissolve it; meanwhile weigh excess Cu(NO 3 ) 2 .3H 2 O 100mg (0.4mmol), add DMF 2mL to dissolve it. The prepared Cu(NO 3 ) 2 The solution was added to the CoTCPP solution, and HNO was added while stirring 3 (1M) 1-4 mL, and finally a mixed solution with red flocs precipitated was obtained. The mixed solution was placed in an oven at 65-100° C. for 5 days to obtain a purple-red powder. Filtration, respectively with DMF, H 2 O and EtOH washed, and dried at room temperature.
[0076] The synthesis of CoTCPP can refer to literature: (a) Lindsey, J.S., H.C.Hsu, and I.C.Schreiman, SYNTHESIS OF TETRAPHENYLPORPHYRINS UNDER VERY MILD CONDITIONS. Tetrahedron Letters, 1986.27(41): 4969-4970. (b) Kumar, A., et al., One-pot general synthesis of metalloporphyrins. Tetrahedron Letters, 2007.48(41): 7287-7290....
Embodiment 2
[0078] Preparation of Embodiment 2 Electrochemical Sensor-I
[0079] (1) Polishing and cleaning of bare glassy carbon electrodes
[0080] Wash the glassy carbon electrode with secondary deionized water and ultrasonically for one minute, then polish it with alumina powder with a diameter of 0.3um for five minutes, wash the grinding cloth and the slurry on the electrode with secondary deionized water, and Put the glassy carbon electrode in the secondary deionized water for one minute, after repeated grinding and cleaning, finally dry the glassy carbon electrode for later use.
[0081] (2) Electrode modification
[0082] Ultrasonic dispersion of 5 mg of CoTCPP-Cu in 400 μL deionized water to form a suspension, 6 μL of the suspension was drop-coated on the surface of the glassy carbon electrode obtained in step (1), and dried; then drop-coated 2 μL of 1% nafion solution on the electrode surface , and dried to obtain the CoTCPP-Cu modified electrode, which is denoted as electric ...
Embodiment 3
[0083] Embodiment 3 Electrochemical sensor-1 is applied to the cyclic voltammetry scanning of hydrogen peroxide detection
[0084] (1) Preparation of hydrogen peroxide standard solution
[0085] Take 84μL of 30% hydrogen peroxide solution, and dilute it to 4mL. That is to prepare 0.2mol L -1 Hydrogen peroxide solution, other concentrations were prepared in the same way.
[0086] (2) Cyclic voltammogram for hydrogen peroxide detection
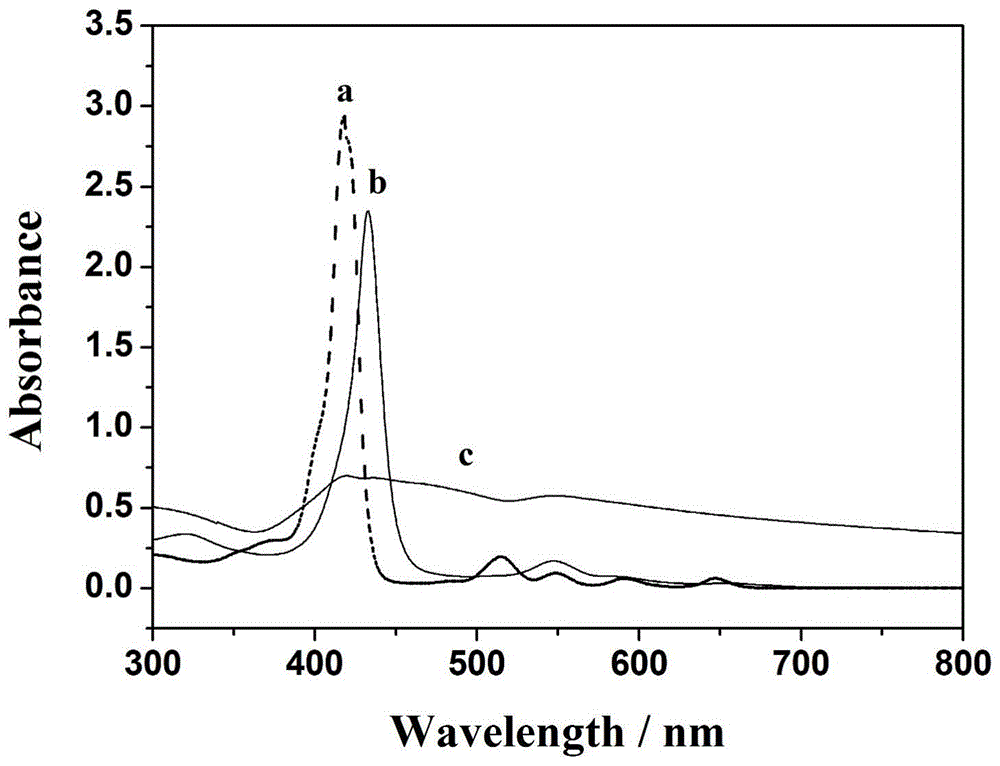
[0087] The C-V curves of the bare electrode and the electrochemical sensor-I with or without hydrogen peroxide in PBS electrolyte at pH = 7, as shown in the attached Figure 5A Shown, a is naked GCE, b is naked GCE plus 0.5mmolL -1 h 2 o 2 , the comparison between a and b shows that the bare GCE has an effect on H 2 o 2 No response. c-d are electrochemical sensor-I adding different concentrations of H 2 o 2 0 and 0.5 mmol L -1 , with the addition of hydrogen peroxide, the response of the reduction current is gradually enhanced, indica...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com