Preparation method of multi-arm acrylate block copolymer
A technology of block copolymer and acrylate, applied in the field of preparation of multi-arm acrylate block copolymer, can solve the problems of high realization difficulty and complex synthesis of hyperbranched macromolecular initiators, and achieves simplified process and realization difficulty, The effect of improved regularity and improved performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
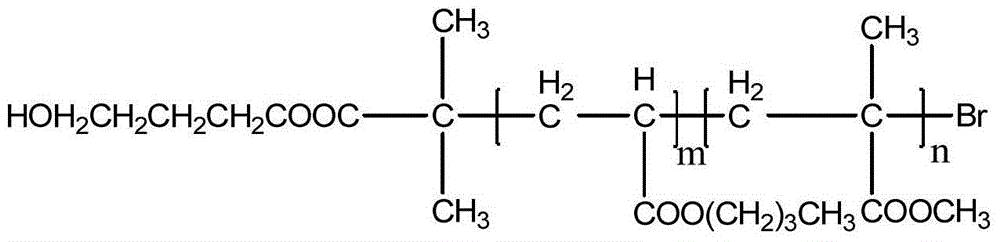
[0025] (1) Preparation of hydroxyl-terminated polybutyl acrylate-b-polymethyl methacrylate (HO-PBA-b-PMMA), the molecular weight is 40600, and the structure is:
[0026]
[0027] Wherein, m=200, n=150.
[0028] Add 60.00 g of butyl acrylate, 0.5604 g of hydroxybutyl α-bromoisobutyrate, CuBr 2 0.04194g and pentamethyldivinyltriamine 0.6490g, Sn(EH) 2 1.517g and 30.00g of solvent toluene, after mixing evenly, bubbling with nitrogen gas, reacting in an oil bath at 70°C for 210min, after the conversion rate reaches 82.7%, weighed 35.16g of methyl methacrylate, 18.00g of toluene and Sn(EH) 2 1.138g was added into the reaction bottle with a syringe, and the reaction was stopped after 255min. The product was passed through a neutral alumina chromatography column to remove the catalyst in the polymer, and methanol was precipitated after rotary evaporation, and then dried in a vacuum oven at 50°C until constant 79 g of the product was obtained with a yield of 83%.
[0029] (2) P...
Embodiment 2
[0033] (1) Preparation of hydroxyl-terminated polyethylacrylate-b-polybutylmethacrylate (HO-PEA-b-PBMA), the molecular weight is 32000, and the structure is:
[0034]
[0035] Wherein, m=200, n=85.
[0036]Add 60.00 g of ethyl acrylate, 0.7173 g of α-bromoisobutyrate 0.7173 g, CuBr 2 0.05369g and pentamethyldivinyltriamine 0.8308g, Sn(EH) 2 1.942g and 30.00g of solvent toluene, after mixing evenly, bubbling with nitrogen gas, reacting in an oil bath at 70°C for 210min, after the conversion rate reaches 85%, 36.00g of methyl methacrylate, 18.00g of toluene and Sn(EH) 2 Add 0.8264g into the reaction bottle with a syringe, stop the reaction after 260min, pass the product through a neutral alumina chromatography column, remove the catalyst in the polymer, and precipitate methanol after rotary evaporation, and then dry it in a vacuum oven at 50°C until constant Weight, to obtain product 75g, yield 78%.
[0037] (2) Preparation of multi-arm PEA-b-PBMA
[0038] NCO / OH=1.1:1 ...
Embodiment 3
[0041] (1) Preparation of hydroxyl-terminated polymethyl acrylate-b-polyisooctyl acrylate (HO-PMA-b-PEHA), the molecular weight is 21000, and the structure is:
[0042]
[0043] Wherein, m=100, n=68.
[0044] Add 60.00 g of methyl acrylate, 1.668 g of hydroxybutyl α-bromoisobutyrate, CuBr 2 0.06251g and pentamethyldivinyltriamine 0.967g, Sn(EH) 2 2.261g and 30.00g of solvent toluene, after mixing evenly, bubbling with nitrogen gas, reacting in an oil bath at 70°C for 250min, after the conversion rate reached 81%, weighed 86.57g of isooctyl acrylate, 43.00g of toluene and Sn (EH) 2 Add 1.525g into the reaction bottle with a syringe, stop the reaction at 70°C for 300min, pass the product through a neutral alumina chromatography column, remove the catalyst in the polymer, and precipitate methanol after rotary evaporation, and then place it in a vacuum oven at 50°C After drying to constant weight, 103 g of the product was obtained with a yield of 70.2%.
[0045] (2) Prepara...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com