Preparation method of Hainantoxin-III
A Hainan tarantula and toxin technology, applied in the field of solid-phase peptide synthesis, can solve the problems of limited research and application, difficulty in obtaining natural toxins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Synthesis of Hainan Tarantula Toxin III Peptide Chain
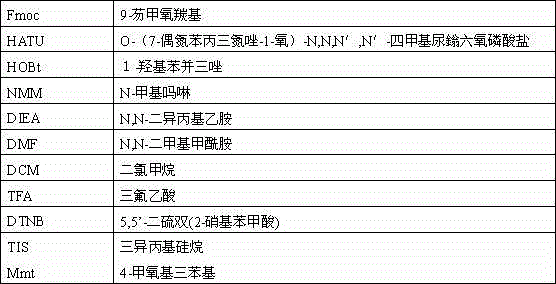
[0027] Weigh 20 grams of Rink Amide resin with a substitution value of 0.5 mmol / g, expand it in DMF for 30 minutes, drain it, add 30 mL of 20% piperidine for deprotection for 15 minutes, drain it, add 30 mL of DMF to wash once, add 20% piperidine 30 ml was deprotected for 10 minutes, washed 5 times with DMF, and completed Fmoc-Leu-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Val-OH, Fmoc-Lys(Boc)- OH, Fmoc-Cys(Trt)-OH, Fmoc-Trp(Boc)-OH, Fmoc-Lys(Boc)-OH, Fmoc-His(Boc)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Ser (tBu)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Cys(Trt)-OH, Fmoc-Ala-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Asn(Trt)-OH, Fmoc- Pro-OH, Fmoc-Cys(Mmt)-OH, Fmoc-Cys(Trt)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Lys(Boc)-OH, Fmoc -Gly-OH, Fmoc-Pro-OH, Fmoc-Thr(tBu)-OH, Fmoc-Cys(Trt)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Gly Coupling of -OH, Fmoc-Phe-OH, Fmoc-Gly-OH, Fmoc-Lys(Boc)-OH, Fmoc-Cys(Mmt)-OH, Fmoc-Gly-OH. The NMM / HATU / HOBt system...
Embodiment 2
[0028] Example 2 Hainan Tarantula Toxin III Peptide Resin Removes Mmt Protection
[0029] Example 1 The synthetic Hainan tarantula toxin III peptide resin was deprotected with 20% piperidine / DMF, washed 5 times with DMF, washed 3 times with DCM, dried with nitrogen, added 600 ml of 3% TFA / DCM solution, and reacted After 10 minutes, remove the solution, wash 5 times with DMF, wash 5 times with DCM, and blow dry with nitrogen.
Embodiment 3
[0030] Example 3 Hainan Tarantula toxin III peptide resin is oxidized by air oxidation to form a pair of disulfide bonds
[0031] Add 1000 milliliters of 0.1 M phosphate buffer solution to the Hainan arachnid toxin III peptide resin obtained in Example 2, and add 1,000 ml of 0.1 M phosphate buffer solution to the pH value of 7.5. During the reaction, the air is ventilated, the reaction temperature is room temperature, and the reaction time is determined according to the oxidation process. After 24 hours of reaction, the peptide resin was taken for detection. The peptide resin was washed with water and then added to the DTNB solution. The end point of the reaction was that the solution did not turn yellow.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com