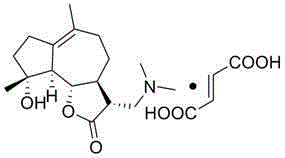
Dimethylamino sphaelactone fumarate and use thereof
A technology of fumarate and dimethylamine, which is applied in the preparation of carboxylates, medical preparations containing active ingredients, and organic active ingredients, etc., can solve the problems of restricting drug research and development, serious hygroscopicity, and instability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment 1 The preparation of dimethylamine containing smilide fumarate
[0012] To a 250 mL round-bottomed flask equipped with mechanical stirring, a thermometer and a reflux condenser, add fumaric acid (1.98 g, 17 mmol), ethyl acetate (2.5 mL), stir, and heat to 77 ° C; Lactone (5.0 g, 17 mmol) and ethyl acetate (15 mL) were mixed uniformly, added dropwise to the reaction, vigorously stirred; after the dropwise addition, the reaction solution was kept at 77 ° C for 3 hours; heating was stopped, and under stirring, Naturally cooled to room temperature, filtered, washed with ethyl acetate, and dried in vacuo for 24 hours to obtain the compound of formula (I), a white solid of 6.3 g, a yield of 90%.
[0013] Prepare the data of the compound of formula (I):
[0014] Molecular formula: C 21 H 31 NO 7
[0015] Molecular weight: 409
[0016] Appearance: white solid powder
[0017] Elemental analysis data:
[0018] element Calculated / % test value / % ...
Embodiment 2
[0023] Example 2 Pharmacological action of dimethylamine containing smilide fumarate
[0024] Form various cancer cells into 2×10 5 / mL cell suspension, added to a 24-well round-bottom cell culture plate, added test compounds, 5 wells for each tested concentration, incubated at 37 degrees, 5% carbon dioxide saturated humidity for 18 hours, using MTT method in enzyme-linked The absorbance measured by the detector at a wavelength of 570 nm refers to the calculation of the inhibitory effect of the compounds of the present invention on the test cancer cells.
[0025]
[0026] Table 1 Inhibitory effects of compounds of formula (I) on various cancer cells
[0027] cell line Compounds of formula (I) HL-60 31.5 K562 22.3 MCF-7 19.5 CNE-1 26.3 CNE-2 26.5 SW620 33.4 A549 28.3 HepG-2 17.7 Ec9706 23.2 SGC7901 14.7 SW1116 37.1 A498 24.1 ASPC-1 31.5 HT-29 19.2 HeLa 29.4 GL15 45.2 ...
Embodiment 3
[0029] Example 3 Stability comparison of compound of formula (I) and compound of formula (II)
[0030] 1. High temperature test
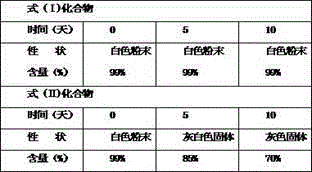
[0031] Take the compound of formula (I) and compound of formula (II) and put them in an open container, put them in a constant temperature box at 60°C for 10 days, take samples at 0 days, 5 days and 10 days, observe the properties, and determine the content.
[0032]
[0033] Table 2 High temperature test results (60℃)
[0034]
[0035] 2. High humidity test
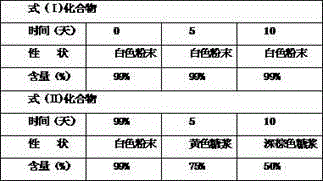
[0036] Take the compound of formula (I) and compound of formula (II) and put them in an open container, place them in a closed container with a relative humidity of 90±5% (saturated potassium nitrate solution), and place them for 10 days. Sampling, observe the properties, and determine the content.
[0037] Table 3 High humidity test results (90±5%)
[0038]
[0039] The results show that the compound of formula (I) is more stable than the compound of formula (II) no matter un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com