Method and kit for detecting human serum hybrid light chain antibody
A kit and human detection technology are applied in the field of clinical laboratory science to achieve the effects of convenient use, strong specificity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Kit for detecting hybrid light chain antibodies in human serum
[0056] 1. Kit composition
[0057] 1. Anti-human lambda light chain antibody coated microwell plate;
[0058] 2. Anti-human kappa light chain antibody labeled with horseradish peroxidase: Abcam Company;
[0059] 3. Washing solution (PBST): PBS solution containing 0.05% Tween-20; (add 500 μL Tween-20 to 1L PBS, mix well)
[0060] 4. Diluent: PBST containing 20% (v / v) calf serum;
[0061] 5. Substrate solution: 3′,3′,5′,5′,-tetramethylbenzidine (TMB);
[0062] 6. Standard reference serum: 20 cases of healthy people with the same volume of mixed serum, the specified concentration of hybrid antibody is 1AU / ml;
[0063] 7. Negative control solution: PBST of 20% calf serum by volume;
[0064] 8. Stop solution: 0.5M sulfuric acid.
[0065] 2. Conditions and methods for preparing anti-human lambda light chain-coated microwell plates:
[0066] A: Microplate: Nunc, 96-well plate, Denmark;
[006...
Embodiment 2
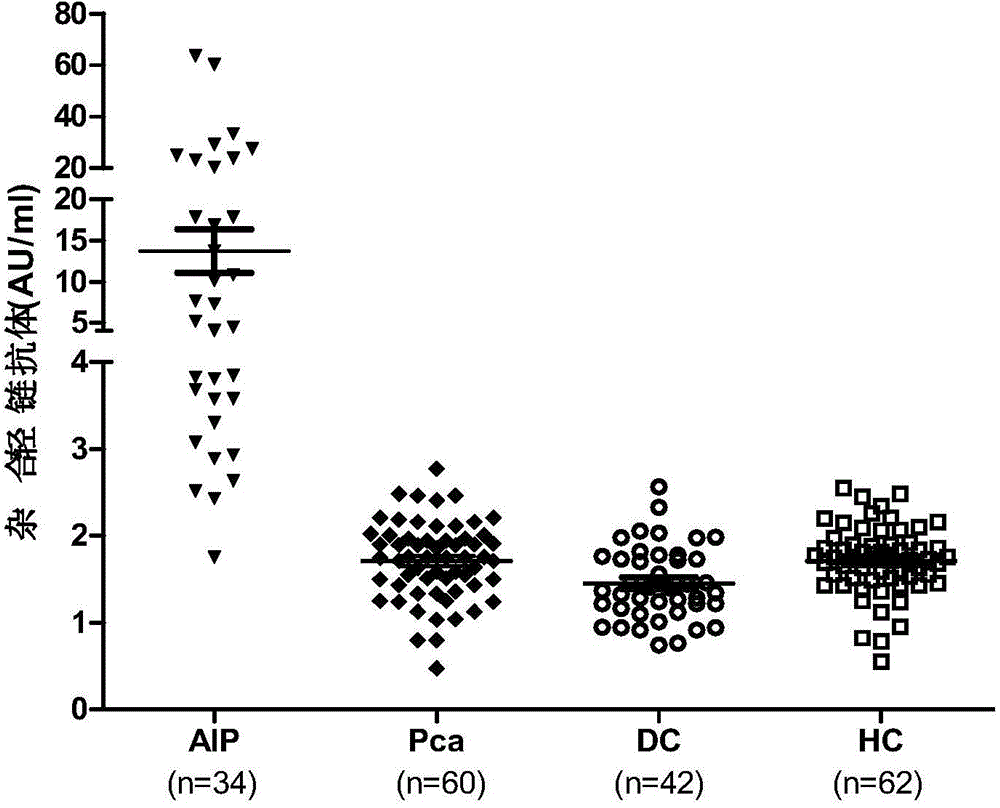
[0072] Example 2: Detection of Human Serum Hybrid Light Chain Antibody
[0073] 1. Materials
[0074] 1. the test kit described in embodiment 1
[0075] 2. Serum: Serum from 34 patients with autoimmune pancreatitis, 60 patients with pancreatic cancer, 42 disease controls (including 23 cases of acute pancreatitis and 19 cases of chronic non-autoimmune pancreatitis), 62 serum samples from healthy people . Stored at -80°C until analysis.
[0076] 2. Method
[0077] 1. Detection of heterozygous light chain antibodies by double-antibody sandwich method
[0078] (1) All materials and reagents were equilibrated to room temperature before measurement;
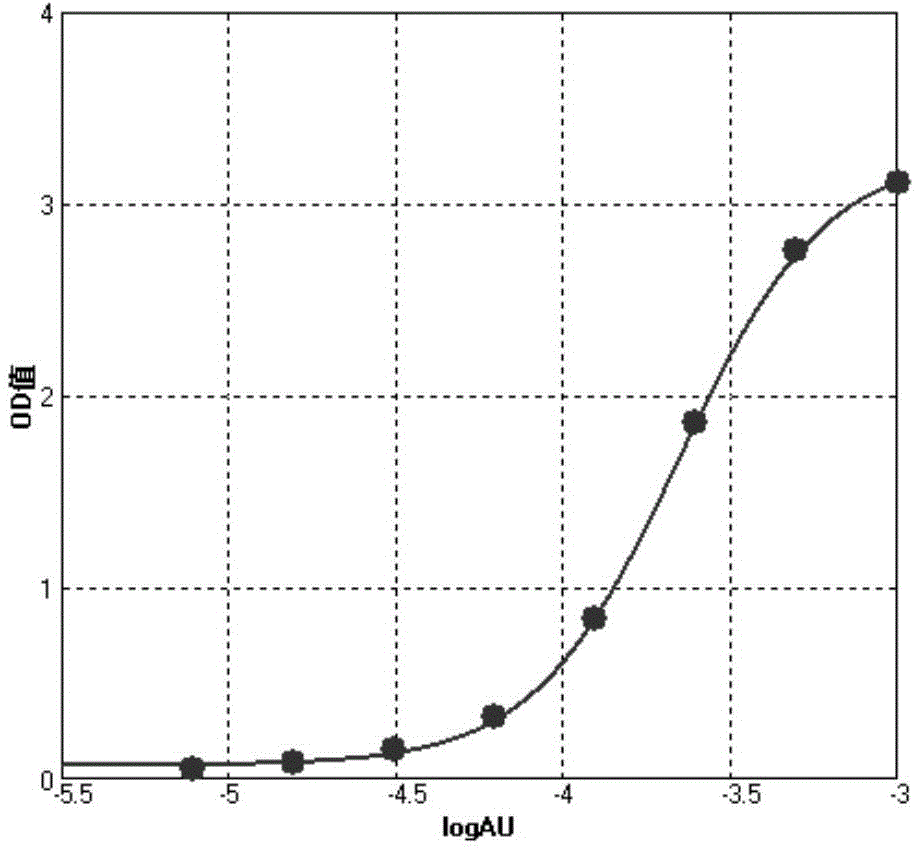
[0079] (2) Dilute the standard serum: Use a sample gun to draw 1 μL of the standard serum provided by the kit and add it to 1 mL of the diluent to prepare a 1AU / uL stock solution, then draw 500 uL and add it to 1 ml of the diluent, and perform 6 consecutive times of 2 times Gradual dilution to prepare standard serum with concentr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap