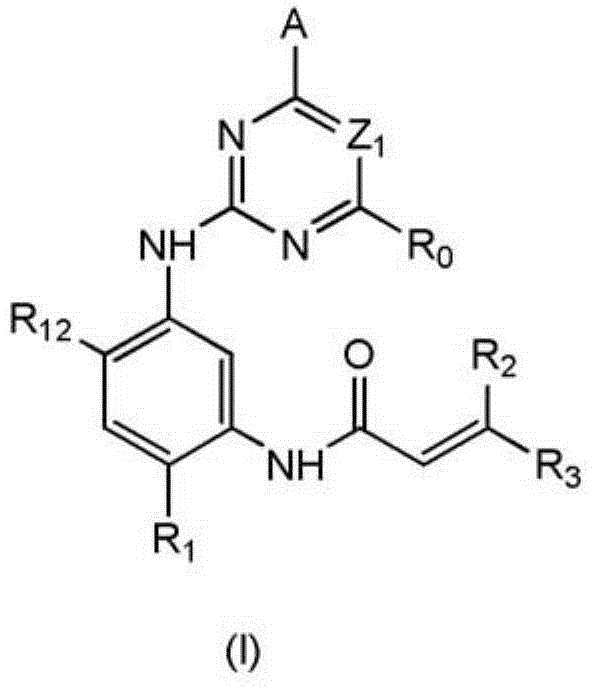
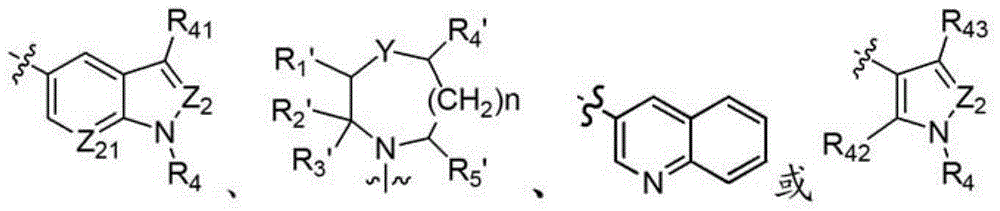
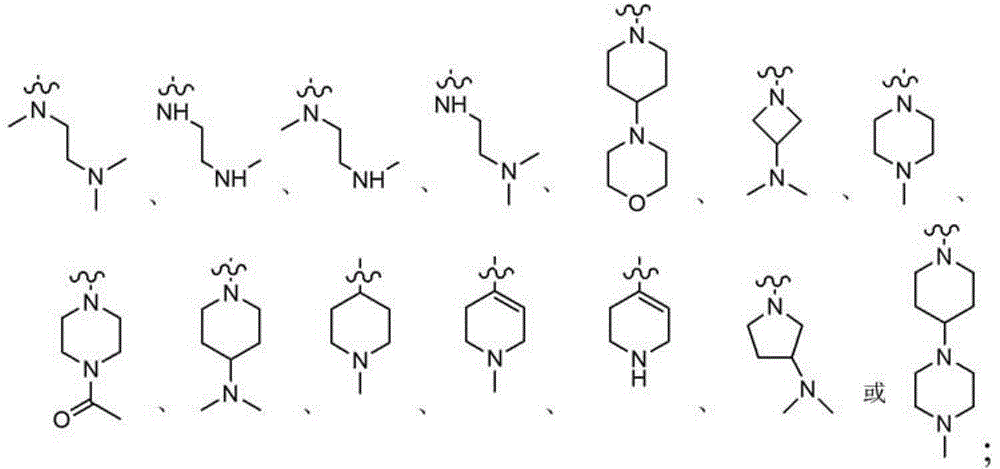
2,4-disubstituted phenyl-1,5-diamine derivatives and use thereof, and pharmaceutical composition and medicinal composition prepared from 2,4-disubstituted phenyl-1,5-diamine derivative
A diamine derivative and a disubstitution technology, applied in the field of medicine, can solve problems such as intolerable toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] N-(5-(5-chloro-4-(1-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrimidin-2-amino)-2-((2-( Dimethylamino)ethyl)methylamino)-4-methoxyphenyl)propionamide (Z-1)
[0164]
[0165] The preparation method of compound 1a:
[0166]
[0167] Step a:
[0168]
[0169] Operation steps: Place the reaction substrate 1 (10.6g, 58mmol) in a 500mL single-port reaction flask, and add a tetrahydrofuran / water (100mL / 60mL) mixed solution to dissolve the substrate. At room temperature, ammonium chloride (15.5 g, 292 mmol) and reduced iron powder (26 g, 467 mmol) were sequentially added to the stirred reaction flask, and then the reaction system was heated to 65 degrees Celsius and kept stirring for 3 hours. The progress of the reaction was detected by TLC. After the substrate was completely reacted, excess iron powder was removed by filtration, and the filter cake was rinsed three times with ethyl acetate. The filtrate was extracted three times with ethyl acetate / water system, the org...
Embodiment 2
[0188] N-(5-(5-chloro-4-(1-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrimidin-2-amino)-2-(4-(di Methylamino)piperidin-1-yl)-4-methoxyphenyl)acrylamide (Z-2)
[0189]
[0190] Step 1: 5-Chloro-N-(4-(4-(dimethylamino)piperidin-1-yl)-2-methoxy-5-nitrophenyl)-4-(1-methyl- 1H-pyrrolo[2,3-b]pyridin-5-yl)pyrimidin-2-amine
[0191] The reaction substrate 5-chloro-N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(1-methyl-1H-pyrrolo[2,3-b]pyridine- 5-yl)pyrimidin-2-amine (260mg, 0.607mmol) and potassium carbonate (251mg, 1.822mmol) were placed in a 25mL single-port reaction flask, and DMF (10mL) was added to partially dissolve the substrate. Then N,N-dimethylaminopiperidine (85.4mg, 0.667mmol) was added and the reaction system was kept heated at 70°C for 3 hours. The progress of the reaction was detected by TLC. After the substrate was completely reacted, the reaction solution was extracted three times with ethyl acetate / water system, the organic layer was separated, washed with water, washed ...
Embodiment 3
[0197] N-(5-(5-chloro-4-(1-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrimidin-2-amino)-4-methoxy-2 -(4-Methylpiperazin-1-yl)phenyl)acrylamide (Z-3)
[0198]
[0199] Step 1: 5-Chloro-N-(2-methoxy-4-(4-methylpiperazin-1-yl)-5-nitrophenyl)-4-(1-methyl-1H-pyrrole And[2,3-b]pyridin-5-yl)pyrimidin-2-amine
[0200] N'-Methylpiperazine (126 mg, 1.26 mmol) and potassium carbonate (261 mg, 1.89 mmol) were added to compound 5-chloro-N-(4-fluoro-2-methoxy-5-nitrophenyl) -4-(1-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrimidin-2-amine (200mg, 0.47mmol) in 4 ml of DMF, vigorously stirred at 100 degrees Celsius for 2h . The progress of the reaction was detected by TLC. After the substrate was completely reacted, 10 milliliters of water was added, extracted three times with ethyl acetate / water system, the organic layer was separated and concentrated under reduced pressure to obtain the compound 5-chloro-N-(2-methoxy -4-(4-methylpiperazin-1-yl)-5-nitrophenyl)-4-(1-methyl-1H-pyrrolo[2,3-b]pyrid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| composition ratio | aaaaa | aaaaa |
| composition ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com