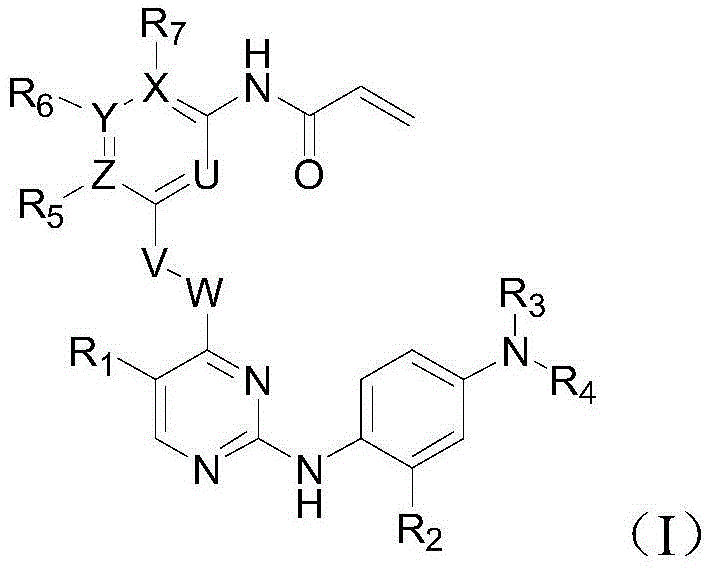
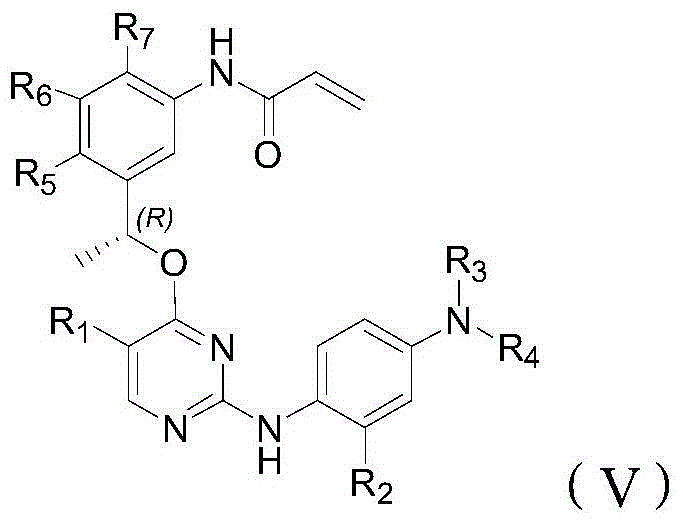
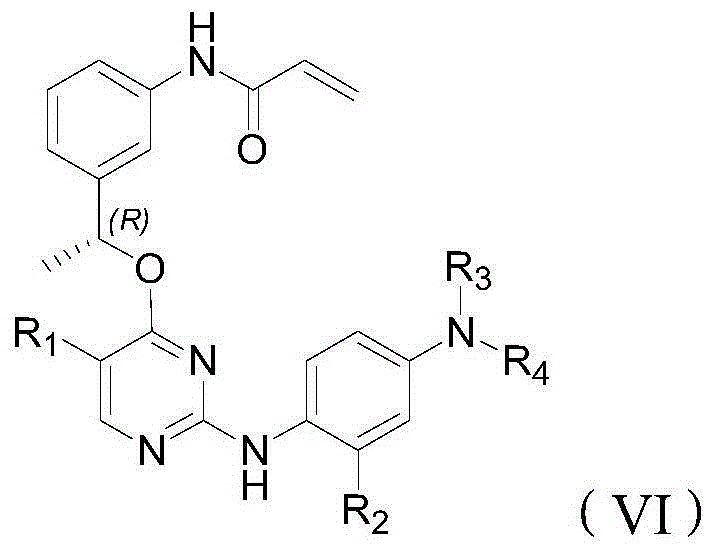
T790M mutant epidermal growth factor receptor (EGFR) inhibitor and application of same in preparation of antitumor drugs
An anti-tumor drug and inhibitor technology, applied in the field of drugs for the treatment of tumors, can solve problems such as loss of inhibitor activity, wild-type cytotoxic side effects, and increased affinity between EGFR and ATP.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1, (R)-N-(3-(1-((2-((4-(4-acetylpiperazin-1-yl)-2-methoxyphenyl)amino)-5-tri Fluoromethylpyrimidin-4-yl)oxy)ethyl)propyl)acrylamide
[0091]
[0092] Synthetic steps:
[0093]
[0094] Step 1: Preparation of tert-butyl 3-acetylphenylcarbamate (compound 2)
[0095] To a solution of 1-(3-aminophenyl)ethanone (compound 1) (8.0 g, 59.3 mmol) in dioxane (100 mL) was added (Boc) 2 O (16.8 g, 77.1 mmol). The obtained reaction solution was reacted at 150° C. for 4 hours, and then concentrated under reduced pressure. The residue was subjected to silica gel column chromatography (PE:EA=8:1 to 4:1) to obtain the target product (12.4 g, 88.6% yield) as a white solid. 1 H NMR (400MHz, CDCl 3 )δ7.95(s,1H),7.66(d,J=7.6Hz,1H),7.60(d,J=7.8Hz,1H),7.37(t,J=7.9Hz,1H),6.88(s, 1H), 2.59(s, 3H), 1.52(s, 9H).
[0096] Step 2: Preparation of (R)-(3-(1-hydroxyethyl)phenyl)carbamate tert-butyl ester (Compound 3)
[0097] (+)-DIP-Cl (5.5 g, 17.0 mmol) was dissolved in anhydro...
Embodiment 2
[0104] Example 2, (S)-N-(3-(1-((2-((4-(4-acetylpiperazin-1-yl)-2-methoxyphenyl)amino)-5-tri Fluoromethylpyrimidin-4-yl)oxy)ethyl)propyl)acrylamide
[0105]
[0106] The synthetic method is as embodiment 1,
[0107] 1 H NMR (400MHz, CDCl 3 )δ8.34(s,1H),7.86(m,2H),7.57(m,3H),7.30(t,J=7.8Hz,1H),7.16(d,J=7.4Hz,1H),6.64– 6.37(m,3H),6.28(m,2H),5.78(d,J=10.3Hz,1H),3.87(s,3H),3.80–3.70(m,2H),3.69–3.54(m,2H) ,3.22–2.98(m,4H),2.19(s,3H),1.66(d,J=6.6Hz,3H).LC-MS:m / z 585(M+H) + .
Embodiment 3
[0108] Example 3, N-(3-(1-((2-((4-(4-acetylpiperazin-1-yl)-2-methoxyphenyl)amino)-5-trifluoromethylpyrimidine -4-yl)oxy)ethyl)propyl)acrylamide
[0109]
[0110] The synthetic method is as embodiment 1,
[0111] 1 H NMR (400MHz, CDCl 3 )δ8.34(s,1H),7.85(m,2H),7.48(m,3H),7.31(t,J=7.8Hz,1H),7.16(d,J=7.6Hz,1H),6.64– 6.37(m,3H),6.27(m,2H),5.79(d,J=10.2Hz,1H),3.86(s,3H),3.82–3.70(m,2H),3.68–3.58(m,2H) ,3.25–2.91(m,4H),2.06(s,3H),1.66(d,J=6.5Hz,3H).LC-MS:m / z 585(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com