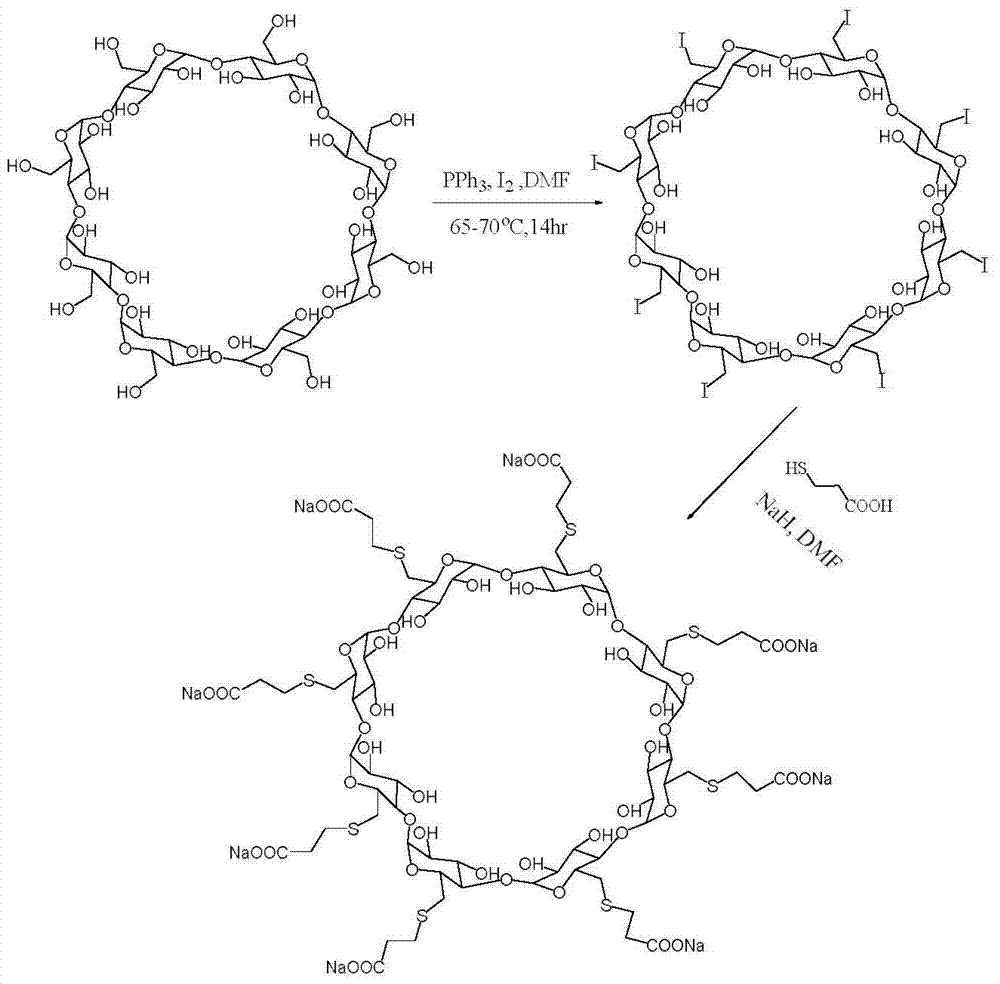
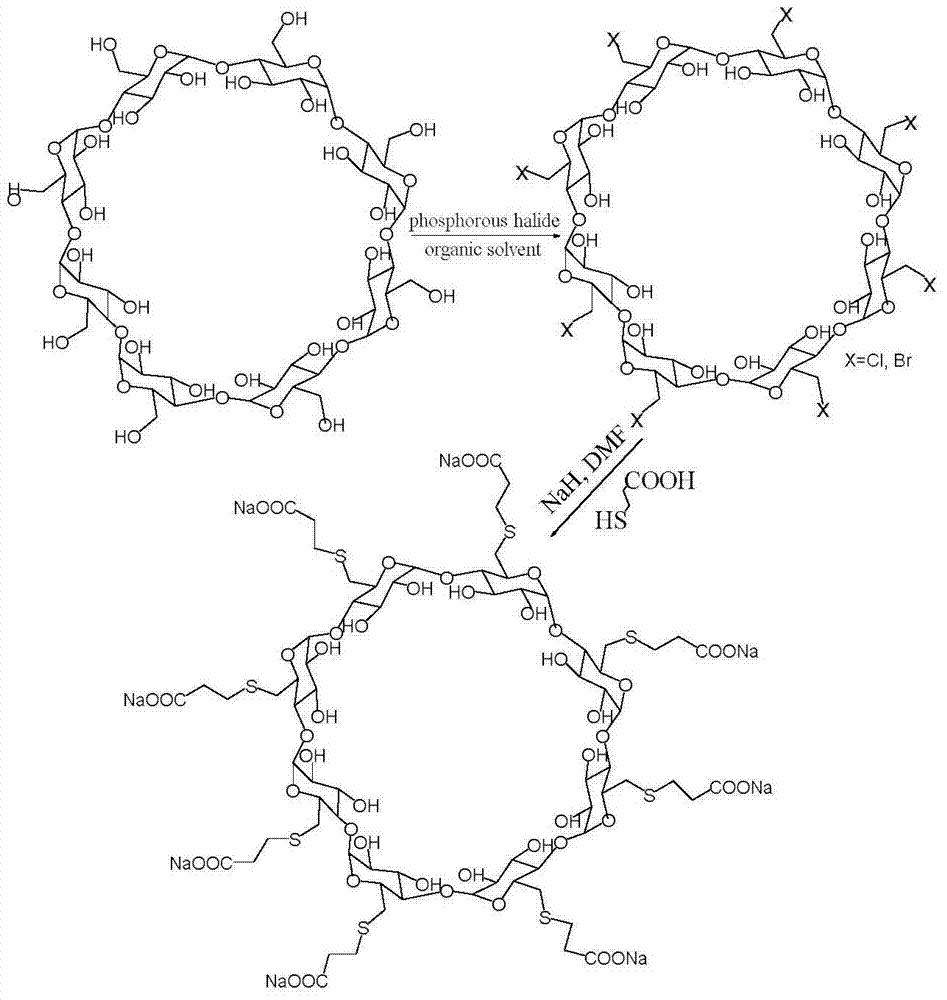
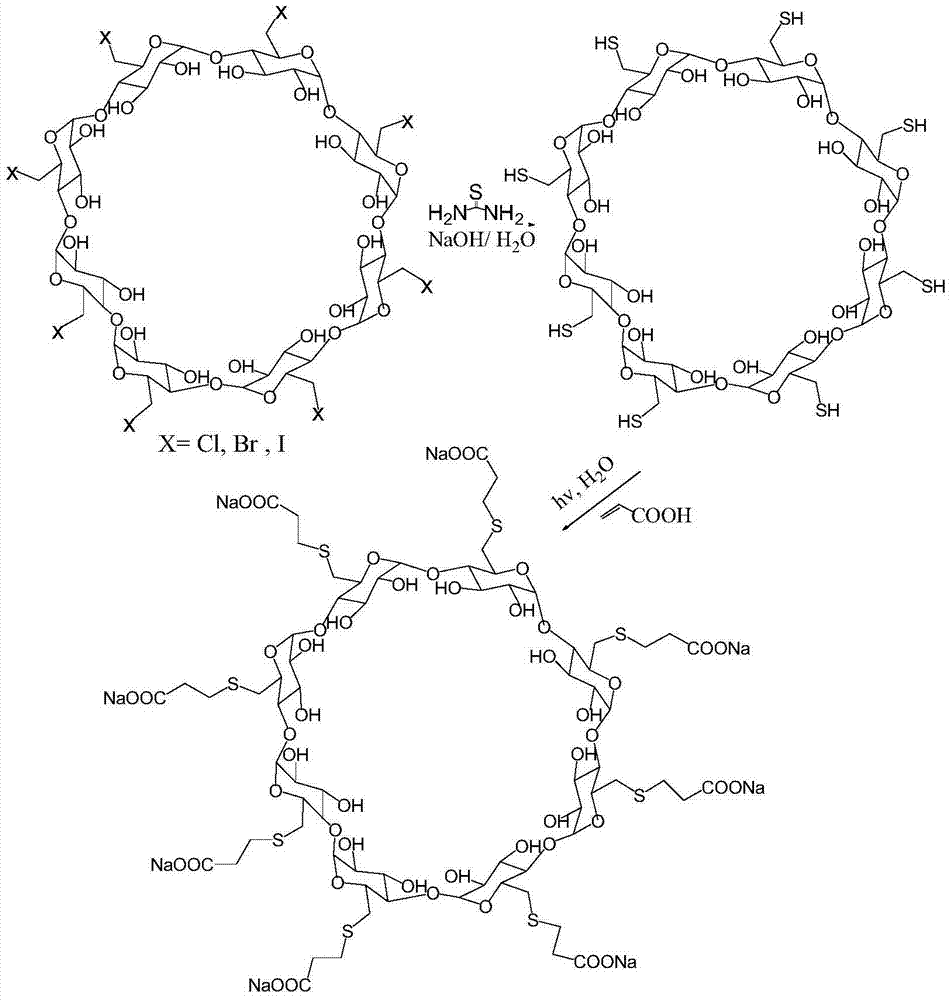
Preparation method for sugammadex sodium
A technology of sugammadex sodium and cyclodextrin, which is applied in the field of drug preparation, can solve the problems of difficult separation of impurities, gelation, and large ethanol consumption, and achieve simple purification process, mild operating environment, and increased yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A preparation method of sugammadex sodium, comprising steps as follows:
[0054] (1) Add 20g (13.9mmol) of 6-perdeoxy-6-perchloro-γ-cyclodextrin and 42g (556mmol) of thiourea into 600ml of N,N-dimethylformamide, stir at 90°C React for 12 hours; first concentrate the reaction solution under reduced pressure until the volume of the reaction solution is 1 / 4 of the volume of N,N-dimethylformamide, and then add ethanol 8 times the volume of the concentrated solution for precipitation. The obtained solid precipitate was added into 750 ml of aqueous sodium hydroxide solution with a concentration of 0.25 mol / L and stirred at 90° C. for 2 hours to obtain a mixed reaction solution. The volume of the liquid was concentrated under reduced pressure to 1 / 4 of the volume of the mixed reaction solution. Adjust the pH to 2, add ethanol 8 times the volume of the reaction solution to the pH-adjusted reaction solution for precipitation, wash the precipitate with ice water, and then recryst...
Embodiment 2
[0060] A preparation method of sugammadex sodium, comprising steps as follows:
[0061] (1) Add 20g (13.9mmol) of 6-perdeoxy-6-perchloro-γ-cyclodextrin and 42g (556mmol) of thiourea into 600ml of N,N-dimethylformamide, stir at 90°C React for 12 hours; first concentrate the reaction solution under reduced pressure until the volume of the reaction solution is 1 / 4 of the volume of N,N-dimethylformamide, and then add ethanol 6 times the volume of the concentrated solution for precipitation. The obtained solid precipitate was added into 750 ml of aqueous sodium hydroxide solution with a concentration of 0.25 mol / L and stirred at 90° C. for 2 hours to obtain a mixed reaction solution. The volume of the liquid was concentrated under reduced pressure to 1 / 4 of the volume of the mixed reaction solution. Adjust the pH to 2, add ethanol 6 times the volume of the reaction solution to the adjusted pH value of the reaction solution for precipitation, wash the precipitate with ice water, and...
Embodiment 3
[0068] With the sugammadex sodium preparation method described in embodiment 1, the difference is:
[0069] In step (1), add 24g of 6-perdeoxy-6-periodo-γ-cyclodextrin and 42g of thiourea into 600ml of N,N-dimethylformamide, heat to 85°C, and react under stirring for 12 hours , first concentrate the reaction solution under reduced pressure until the volume of the reaction solution is 1 / 4 of the volume of N,N-dimethylformamide, and then add ethanol 9 times the volume of the concentrated solution for precipitation. The obtained solid precipitate was added into 750 ml of aqueous sodium hydroxide solution with a concentration of 0.2 mol / L and stirred at 85° C. for 2 hours to obtain a mixed reaction solution. The volume of the liquid was concentrated under reduced pressure to 1 / 4 of the volume of the mixed reaction solution. Adjust the pH to 2, add ethanol 9 times the volume of the reaction solution to the pH-adjusted reaction solution for precipitation, wash the precipitate with i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com