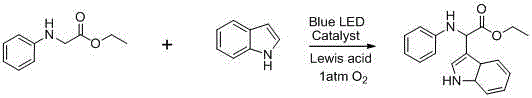Synthesis method of glycine derivatives
A technology of glycine derivatives, which is applied in the field of preparation of glycine derivatives, can solve the problems of heavy metal pollution, inability to use large-scale production, expensive catalysts, etc., achieve fast reaction speed, wide application range of substrates, and easy access to catalysts Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
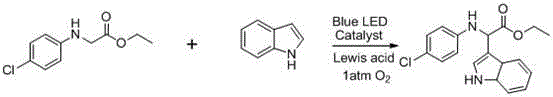
Embodiment 2
[0036] Reaction equation:
[0037]
[0038] where the Lewis acid is FeCl 2 , the solvent is acetonitrile, the catalyst EosinY,
[0039] Proceed as follows:
[0040] In a dry reaction tube, sequentially add 0.1mmol of glycine ester compound, 0.12mmol of indole, 0.005mmol of EosinY, FeCl 2 0.01 mmol.
[0041] Introduce oxygen at an atmospheric pressure, and irradiate with a blue LED for 40 hours. After the reaction, pass through a diatomaceous earth sand core, and remove the solvent under reduced pressure. The product is subjected to column chromatography to obtain the target product (petroleum ether / ethyl acetate = 40 / 1).
[0042] Yield 93%;
[0043]
[0044] 1 H NMR (400 MHz, CDCl 3) δ 8.19 (s, 1H), 7.82 (d, J = 7.8 Hz, 1H), 7.31 (d, J = 8.0 Hz, 1H), 7.20 (dd, J = 15.9, 8.5 Hz, 2H), 7.15 (s, 1H), 6.96 (d, J = 8.1 Hz, 2H), 6.56 (d, J = 8.2 Hz, 2H), 5.36 (s, 1H), 4.62 (s, 1H), 4.23 – 4.08 (m, 2H), 2.21 (s, 3H), 1.20 (t, J = 7.1 Hz, 3H). 13 C NMR (101 M...
Embodiment 3
[0047] Reaction equation:
[0048]
[0049] where the Lewis acid is FeCl 2 , the solvent is acetonitrile, the catalyst EosinY,
[0050] Proceed as follows:
[0051] In a dry reaction tube, sequentially add 0.1mmol of glycine ester compound, 0.12mmol of indole, 0.005mmol of EosinY, FeCl 2 0.01 mmol.
[0052] Introduce oxygen at an atmospheric pressure, and irradiate with a blue LED for 40 hours. After the reaction, pass through a diatomaceous earth sand core, and remove the solvent under reduced pressure. The product is subjected to column chromatography to obtain the target product (petroleum ether / ethyl acetate = 40 / 1).
[0053] Yield 89%;
[0054]
[0055] 1 H NMR (400 MHz, CDCl 3 ) δ 8.22 (s, 1H), 7.80 (d, J = 7.8 Hz, 1H), 7.33 (d, J = 8.0 Hz, 1H), 7.24 – 7.20 (m, 1H), 7.20 – 7.15 (m, 1H), 7.14 (s, 1H), 7.06 (d, J = 8.7 Hz, 2H), 6.52 (d, J = 8.8 Hz, 2H), 5.33 (s, 1H), 4.79 (s, 1H), 4.26 – 4.08 (m, 2H), 1.20 (t, J = 7.1 Hz, 3H). 13 C NMR (101 MHz, CD...
Embodiment 4
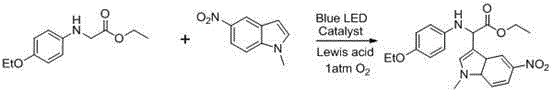
[0058] Reaction equation:
[0059]
[0060] where the Lewis acid is FeCl 2 , the solvent is acetonitrile, the catalyst EosinY,
[0061] Proceed as follows:
[0062] In a dry reaction tube, add glycine ester compound 0.1mmol, 5-nitroindole 0.12mmol, EosinY 0.005mmol, FeCl 2 0.01 mmol.
[0063] Introduce oxygen at an atmospheric pressure, and irradiate with a blue LED for 40 hours. After the reaction, pass through a diatomaceous earth sand core, and remove the solvent under reduced pressure. The product is subjected to column chromatography to obtain the target product (petroleum ether / ethyl acetate = 40 / 1).
[0064] Yield 90%;
[0065]
[0066] 1 H NMR (400 MHz, CDCl 3 ) δ 8.85 (s, 1H), 8.81 (s, 1H), 8.12 (d, J = 8.7 Hz, 1H), 7.42 – 7.32 (m, 2H), 7.15 (t, J = 7.4 Hz, 2H), 6.75 (t, J = 7.0 Hz, 1H), 6.63 (d, J = 7.7 Hz, 2H), 5.42 (s, 1H), 4.91 (s, 1H), 4.31 – 4.13 (m, 2H), 1.24 (t, J = 7.0 Hz, 3H). 13 C NMR (101 MHz, CDCl 3 ) δ 172.02 (s), 146.14 (s), 141...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com