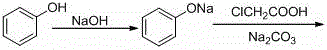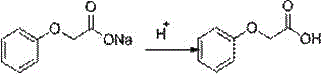A kind of continuous synthesis method of phenoxyacetic acid
A technology for the synthesis of phenoxyacetic acid, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as increased consumption of chloroacetic acid, reduced selectivity and yield of chemical synthesis reactions, and meets equipment requirements Low, reduce energy consumption, improve the effect of yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] 1) Add 56.7g of chloroacetic acid to a clean 500mL four-necked bottle and slowly dissolve the solid Na with 100g of water under stirring 2 CO 3 26.5g was dissolved and slowly added to chloroacetic acid to obtain a colorless sodium chloroacetate solution for use;
[0025] 2) Add 47g of solid phenol and 60g of water to a clean 1000mL four-necked bottle, and slowly add 40% sodium hydroxide solution (24g of sodium hydroxide + 36g of purified water) dropwise under nitrogen protection to control the temperature below 35°C;
[0026] 3) Add sodium phenate aqueous solution and sodium chloroacetate aqueous solution at a molar ratio of 1.0: 1.0 to a constant pressure funnel with a heating sleeve at the same time, and react at about 95°C to keep the retention time at 15 minutes. The reaction solution is then Overflow into a three-necked flask filled with dilute hydrochloric acid at 15°C, adjust pH=5 with dilute hydrochloric acid, then stir at 25°C for 1 hour, and filter with sucti...
example 2
[0028] 1) Add 56.7 g of chloroacetic acid to a clean 500 mL four-necked bottle and slowly dissolve the solid Na with 100 g of water under stirring. 2 CO 3 26.5g was dissolved and slowly added to chloroacetic acid to obtain a colorless sodium chloroacetate solution for use;
[0029] 2) Add 47 g of solid phenol and 60 g of water to a clean 1000 mL four-necked bottle, and slowly add 40% sodium hydroxide solution (24 g of sodium hydroxide + 36 g of purified water) dropwise under nitrogen protection to control the temperature at 35 Below ℃;
[0030] 3) The sodium phenate aqueous solution and the sodium chloroacetate aqueous solution were added dropwise at the same time in a constant pressure funnel with a heating sleeve at a molar ratio of 1.0: 1.05, and reacted at about 95°C to keep the retention time at 12 min. Then overflowed into a three-necked flask filled with dilute sulfuric acid at 20°C, adjusted to pH=6 with dilute sulfuric acid, then stirred at 30°C for 1 h, and filter...
example 3
[0032] 1) Add 56.7 g of chloroacetic acid to a clean 500 mL four-necked bottle and slowly dissolve the solid Na with 100 g of water under stirring. 2 CO 3 26.5 g was dissolved, and it was slowly added to chloroacetic acid to obtain a colorless sodium chloroacetate solution for use;
[0033] 2) Add 47 g of solid phenol and 60 g of water to a clean 1000 mL four-necked bottle, and slowly add 40% sodium hydroxide solution (24 g of sodium hydroxide + 36 g of purified water) dropwise under nitrogen protection to control the temperature Below 35°C;
[0034] 3) Add sodium phenate aqueous solution and sodium chloroacetate aqueous solution at a molar ratio of 1.0: 1.0~1.03 to a constant pressure funnel with a heating sleeve at the same time, and react at about 95°C to keep the retention time at 10 minutes. The liquid then overflowed into a three-neck flask filled with dilute hydrochloric acid at 18°C, adjusted to pH=5.5 with dilute hydrochloric acid, then stirred at 27°C for 1 h, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com