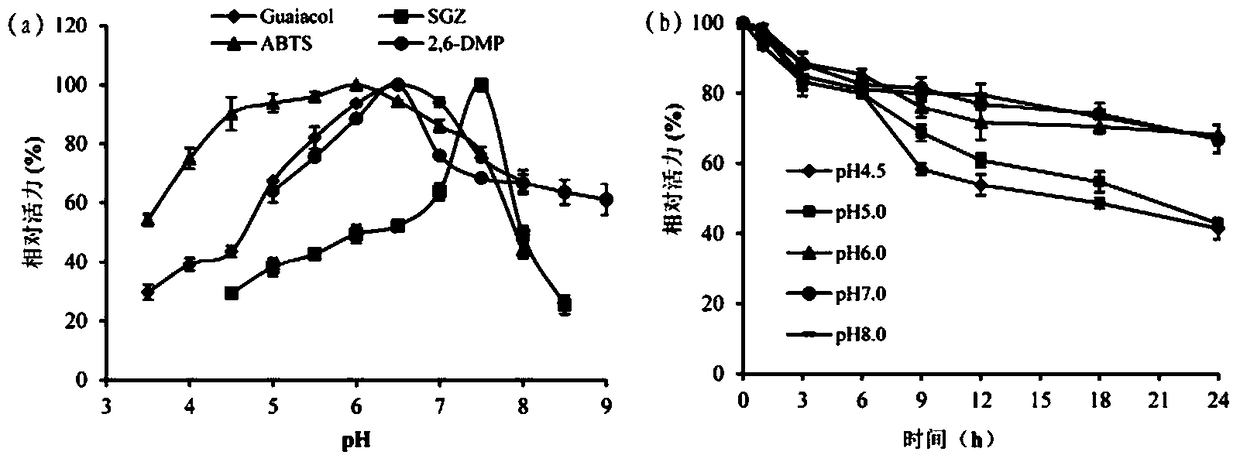
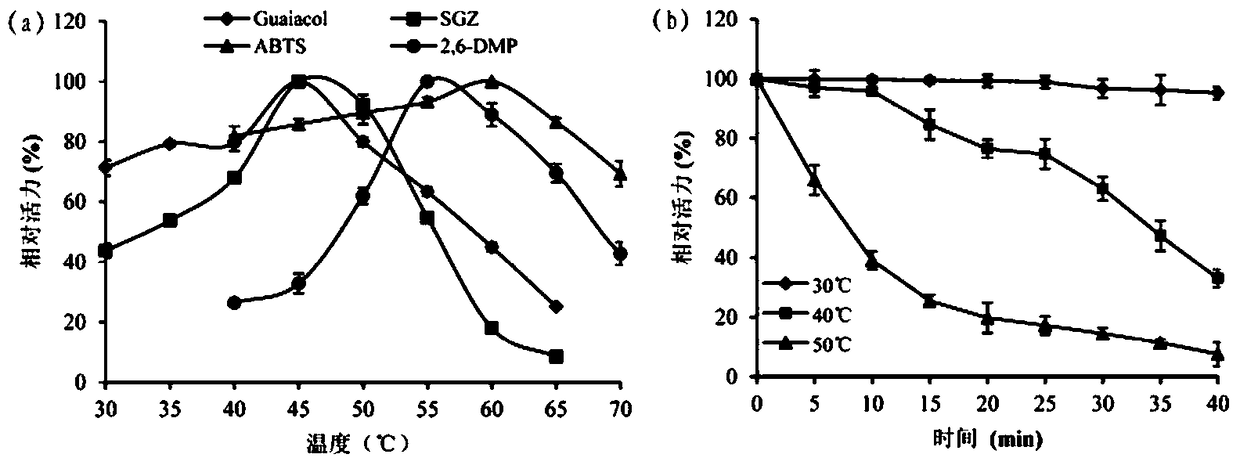
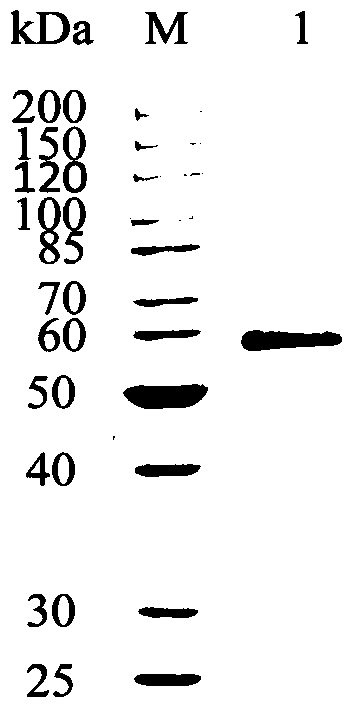A kind of cocoa arbuscularis laccase and its engineering bacteria, recombinant laccase and application
A technology of engineering bacteria and laccase, applied in the field of environmental biology and biology, can solve the problems of easy loss of activity, sensitivity to chloride ions, application limitation of fungal laccase, etc., and achieve the effect of good pH stability and high tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of Novel Laccase Gene
[0028] Based on the American National Center for Biotechnology Information (NCBI, http: / / www.ncbi.nlm.nih.gov / ) GenBank accession number: AFD97050 amino acid sequence based on the codon preference of Pichia pastoris The novel laccase gene was optimized to obtain a new type of laccase gene, whose sequence is shown in SEQ ID NO.2. It was synthesized through the whole gene of Shanghai Jierui Bioengineering Co., Ltd., and the synthesized gene was cloned in the pUC57 plasmid (purchased from Shanghai Jierui Bioengineering Co., Ltd. ), the plasmid pUC57-lacMP was obtained.
Embodiment 2
[0030] Construction of Recombinant Plasmid pPICZαA-6AA-LacMP Containing Novel Laccase Gene and the Strain E.coliTop10 / pPICZαA-6AA-LacMP Carrying the Plasmid
[0031] Design PCR primers according to the nucleotide sequence of the novel laccase gene: forward primer PMPF (SEQ ID NO: 3): 5'-G GAATTC GCTATTGGACCAGTTGCT-3' (the EcoRI restriction site is underlined) and reverse primer PMPR (SEQ ID NO: 4): 5'-ATTT GCGGCCGC CAAATCAGA GGCTGGAGTAG-3' (the underline is the NotI restriction site). Design PCR primers according to the nucleotide sequence of the α signal peptide of pPICZαA: forward primer α-factor-6AAF (SEQ ID NO:5): 5'-TA T TCGAA ATGAGATTTCCTTCAATTTTTACT-3' (the underline is the BstBI restriction site) and reverse primer α-factor-6AAR (SEQ ID NO:6): 5'-G GAATTC AAACTCAGCCTCAGTCTCAGCTTCAGCCTCTCTTTTCTCG-3' (the underline is the EcoRI restriction site, and also contains the nucleotide sequence encoding 6 amino acids (ETEAEF)).
[0032] Using the plasmid pUC57-lacMP in E...
Embodiment 3
[0040] Construction of a Novel Laccase-producing Strain Pichia pastoris X33 / pPICZαA-6AA-LacMP and Expression of Recombinant Laccase
[0041] The recombinant plasmid pPICZαA-6AA-LacMP was linearized by PmeI single enzyme digestion and purified, and then electrotransformed into Pichiapastoris X33 (purchased from Invitrogen Yingjie Life Technology Co., Ltd., USA) competent cells, and the YPD plate (1% yeast extract; 2% Tryptone; 2% glucose; 1.5% agar powder; containing 100 μg / L zeocin) for positive transformant screening. The transformant on the YPD plate was used as a template, and PMPF (SEQ ID NO: 3) and PMPR (SEQ ID NO: 4) were used as primers for PCR identification, and the correct transformant identified by PCR was inoculated on the MM plate (1.34% YNB; 4 ×10 -5 % Biotin; 0.5% Methanol; 1.5% Agar Powder; Contains 0.2mM ABTS and 0.1mM CuSO 4 ) for activity identification, the transformant with a dark green reaction circle around the colony is the recombinant Pichia pastoris...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com