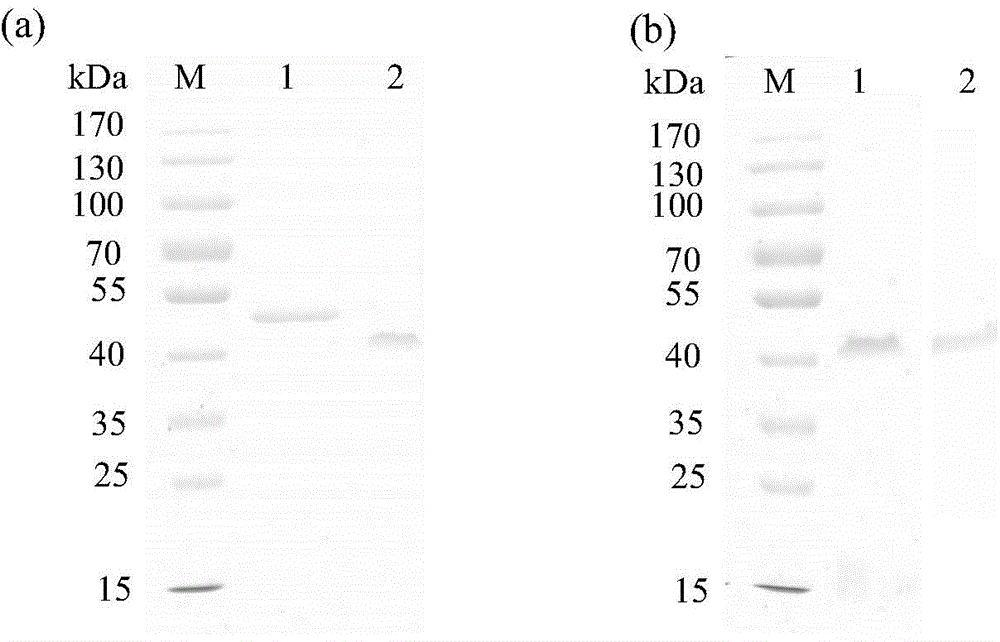
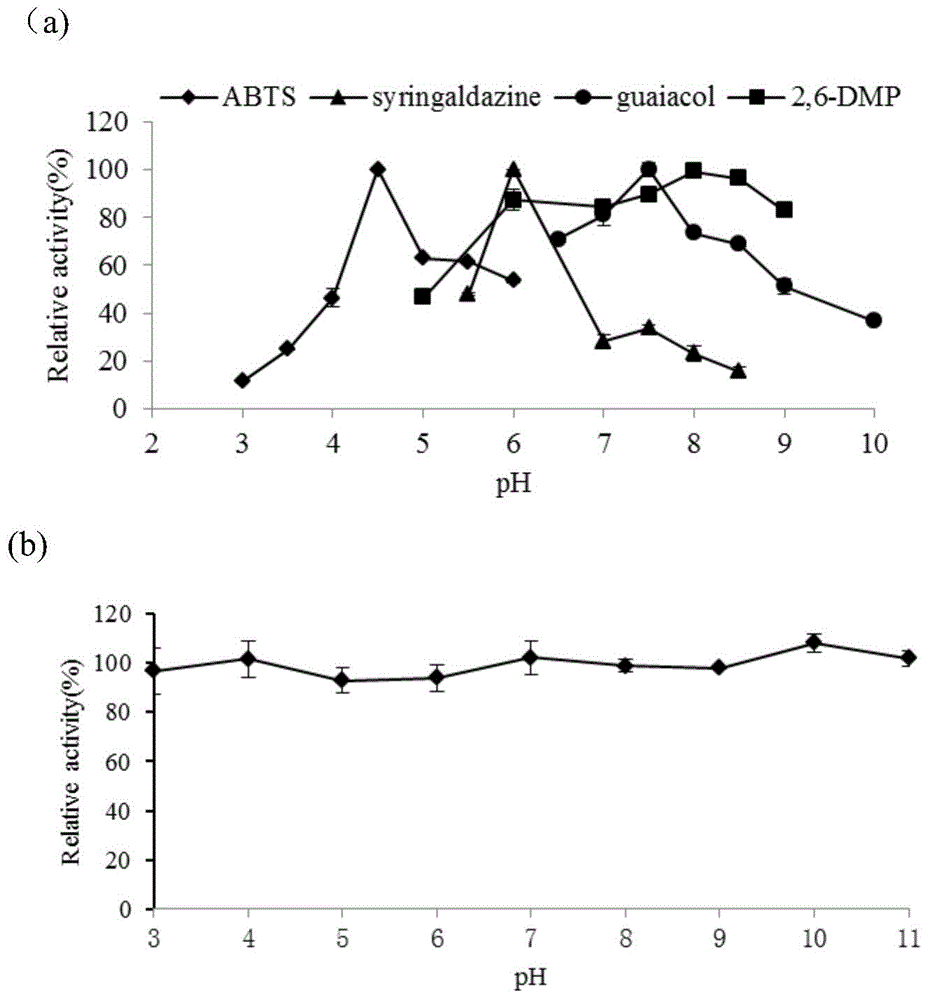
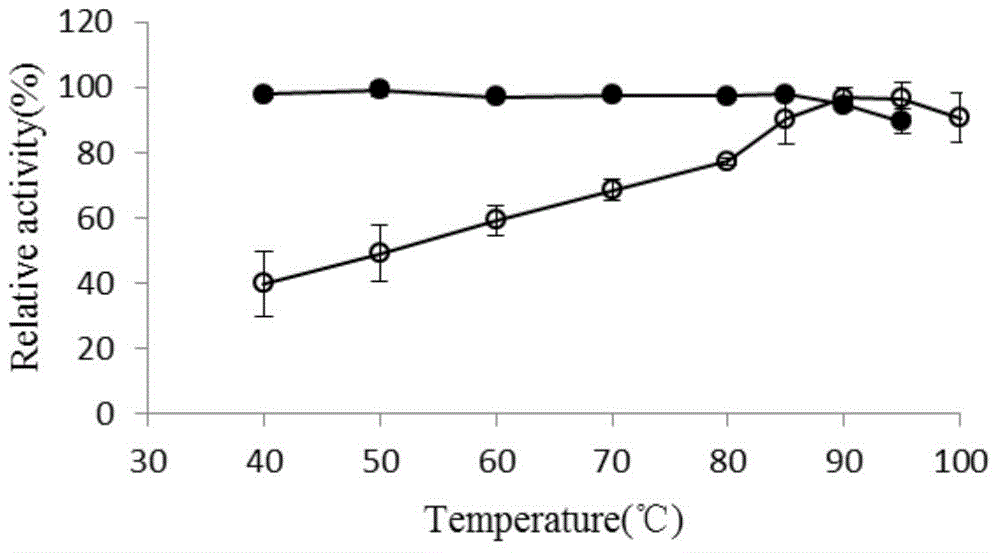
Thermus thermophilus laccase (benzenediol: oxygen oxidoreductases), engineering bacteria, recombinant laccase and use of recombinant laccase
A Thermus thermophilus, engineering bacteria technology, applied in application, genetic engineering, recombinant DNA technology and other directions, can solve problems such as high cost and poor effect, and achieve good heat and alkali resistance, high tolerance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of Novel Laccase Gene
[0030] Based on the GenBand: YP_005641270.1 amino acid sequence published on the National Center for Biotechnology Information (NCBI, http: / / www.ncbi.nlm.nih.gov / ), the sequence is based on the codon preference of Pichia pastoris Optimization was carried out to obtain a novel laccase gene whose sequence is shown in SEQ ID NO.1, which was synthesized by a biotechnology company (Nanjing Kingsray Biotechnology Co., Ltd.), and the synthesized gene was cloned in the pUC57 plasmid (purchased from Kings Rui Biological Technology Co., Ltd.) to obtain the plasmid pUC57-lacTT.
Embodiment 2
[0032] Construction of Recombinant Plasmid pHKFA1-lacTT Containing Novel Laccase Gene and E.coli Top10 / pHKFA1-lacTT Carrying the Plasmid
[0033] Design PCR primers according to the nucleotide sequence of the novel laccase gene: forward primer TTF: 5'-G GAATTC AATACCGATAGAAGAACCCT-3' (underlined EcoRI restriction site) and reverse primer TTR: 5'-ATTT GCGGCCGC TTAGTGGTGGTGGTGGTGGTGGTGGTG TCCGACTTCCAAAACTCC-3' (the underline is the NotI restriction site, which also contains 8×His tag and stop codon).
[0034] The plasmid pUC57-lacTT in Example 1 was used as a template, and TTF and TTR were used as primers for PCR amplification. The gel-recovered and purified PCR product was double-digested with restriction endonucleases EcoRI and NotI, and ligated with the plasmid pHKFA1 that had also been double-digested with EcoRI and NotI with T4 ligase overnight at 16°C, and the ligated product was chemically transformed into E.coli Top10 (purchased from Invitrogen Life Technology Co., L...
Embodiment 3
[0036] Construction of a Novel Laccase-producing Strain Pichia pastoris GS115 / pHKFA1-lacTT and Expression of Recombinant Laccase
[0037]The recombinant plasmid pHKFA1-lacTT was linearized and purified by Kpn2I single enzyme digestion, and then electrotransformed into Pichia pastoris GS115 (purchased from Invitrogen Life Technologies Co., Ltd., USA) competent cells, and positive transformants were screened on MD plates. The transformant screened on the MD plate was used as a template, and TTF and TTR were used as primers for PCR identification. The correct transformant identified by PCR was initially identified as a new laccase-producing strain Pichia pastoris GS115 / pHKFA1-lacTT.
[0038] Pick 3 transformants identified correctly by PCR and inoculate them into BMGY medium, and culture them at 30°C and 250rpm until OD 600 Close to 6.0, collect the cells by centrifugation at 6000rpm, 4°C for 5min, and then resuspend the collected cells in BMMY medium (containing 0.1mM CuSO 4 ) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com