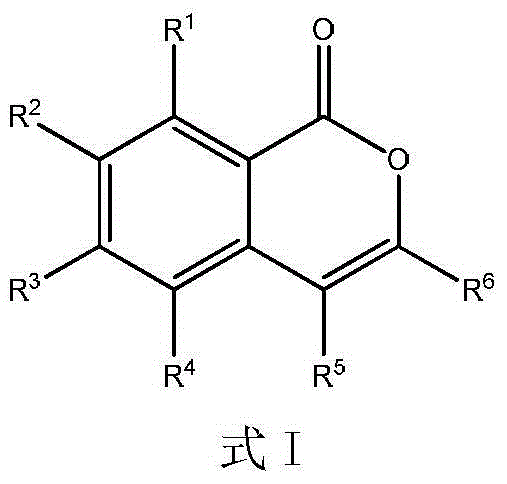
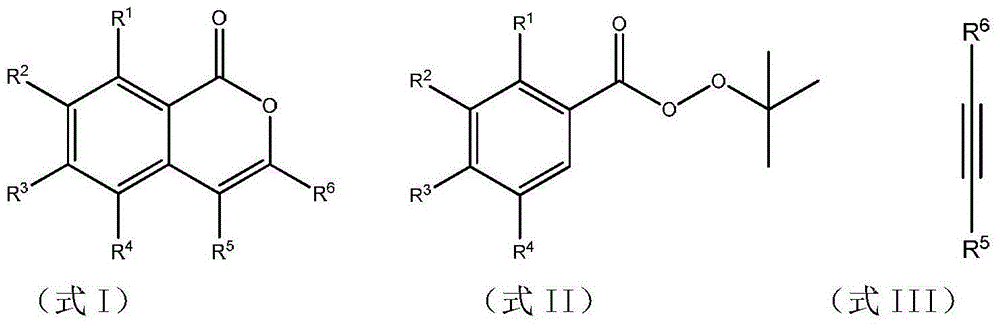
Multi-substituent isocoumarin derivative and preparation method thereof
An isocoumarin, multi-substitution technology, applied in the direction of organic chemistry, can solve the problems of low reaction selectivity, unfriendly environment, narrow substrate range, etc., to achieve strong reaction specificity, green post-processing, substrate wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
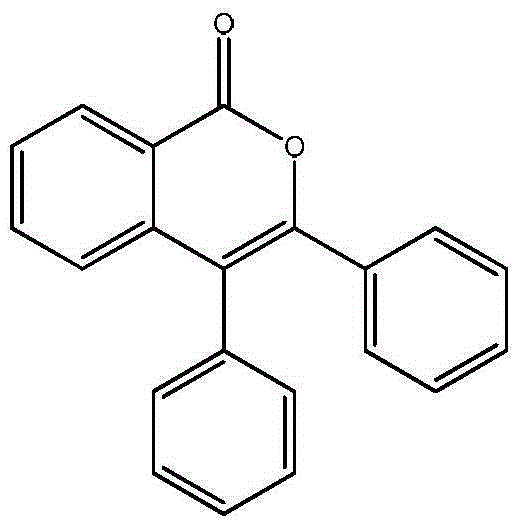
[0023] Preparation of 3,4-diphenyl-1H-isochromen-1-one
[0024]
[0025] 0.5 mmol of tert-butyl peroxybenzoate, 0.75 mmol of 1,2-tolueneacetylene, 0.005 mmol of dichloro(pentamethylcyclopentadienyl) rhodium (III), 0.15 mmol of cesium pivalate, and 0.15 mmol of pivalate Add 0.5 mmol of the acid to a nitrogen-filled Slinky tube, place it in an oil bath at 80°C, and react for 18 hours. Remove the reaction from the heat source and cool to room temperature. The reaction solution was concentrated and purified by column chromatography to obtain 141 mg of the target product with a yield of 95%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.41(dd,J=7.9,1.2Hz,1H),7.68–7.60(m,1H),7.55–7.49(m,1H),7.45–7.37(m,3H),7.36–7.30(m, 2H),7.29–7.25(m,2H),7.24–7.16(m,4H). 13 C NMR (101MHz, CDCl 3 ) δ 162.4, 151.1, 139.0, 134.8, 134.5, 133.1, 131.4, 129.7, 129.4, 129.2, 129.1, 128.3, 128.2, 128.0, 125.5, 120.6, 117.0.
Embodiment 2
[0027] Preparation of 3,4-bis(4-methoxyphenyl)-1H-isochromen-1-one
[0028]
[0029] 0.5mmol of tert-butyl peroxybenzoate, 0.75mmol of 1,2-bis(4-methoxyphenyl)acetylene, 0.005mmol of dichloro(pentamethylcyclopentadienyl)rhodium (III), and Add 0.15 mmol of cesium valerate and 0.5 mmol of pivalic acid to a nitrogen-filled Schlinker tube, place in an oil bath at 80° C., and react for 18 hours. Remove the heat source from the reaction and cool to room temperature. The reaction solution was concentrated and purified by column chromatography to obtain 122 mg of the target product with a yield of 68%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.38(d, J=7.8Hz, 1H), 7.62(t, J=7.7Hz, 1H), 7.48(t, J=7.5Hz, 1H), 7.30(d, J=8.7Hz, 2H) ,7.18(dd,J=12.0,8.3Hz,3H),6.97(d,J=8.4Hz,2H),6.73(d,J=8.7Hz,2H),3.86(s,3H),3.77(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ 162.6, 160.0, 159.5, 151.0, 139.6, 134.6, 132.5, 130.8, 129.6, 127.8, 126.8, 125.6, 125.3, 120...
Embodiment 3
[0031] Preparation of 3,4-bis(4-methylphenyl)-1H-isochromen-1-one
[0032]
[0033] 0.5mmol of tert-butyl peroxybenzoate, 0.75mmol of 1,2-bis(4-methylphenyl)acetylene, 0.005mmol of dichloro(pentamethylcyclopentadienyl) rhodium (III), pentapentyl Add 0.15 mmol of cesium acid and 0.5 mmol of pivalic acid to a nitrogen-filled Schlinker tube, place in an oil bath at 80°C, and react for 18 hours. Remove the heat source from the reaction and cool to room temperature. The reaction solution was concentrated and purified by column chromatography to obtain 134 mg of the target product with a yield of 82%. 1 H NMR (400MHz, CDCl 3 )δ8.40(d, J=7.8Hz, 1H), 7.62(t, J=7.6Hz, 1H), 7.50(t, J=7.5Hz, 1H), 7.27–7.22(m, 4H), 7.20( d, J=8.1Hz, 1H), 7.14(d, J=7.9Hz, 2H), 7.01(d, J=8.1Hz, 2H), 2.42(s, 3H), 2.29(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ 162.4, 151.0, 139.3, 139.0, 137.8, 134.5, 131.4, 131.1, 130.2, 129.8, 129.5, 129.1, 128.6, 127.8, 125.3, 120.4, 116.3,, 21.4, 21.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com