Ferrocene-quinolone amide compound as well as preparation method and application thereof
A quinolone amide, ferrocene technology, applied in chemical instruments and methods, metallocene, organic chemistry and other directions, can solve the problems such as the biological activity of ferrocene-quinolone amide compounds that have not yet been seen, and achieve a simple and easy-to-operate synthesis method. , obvious inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
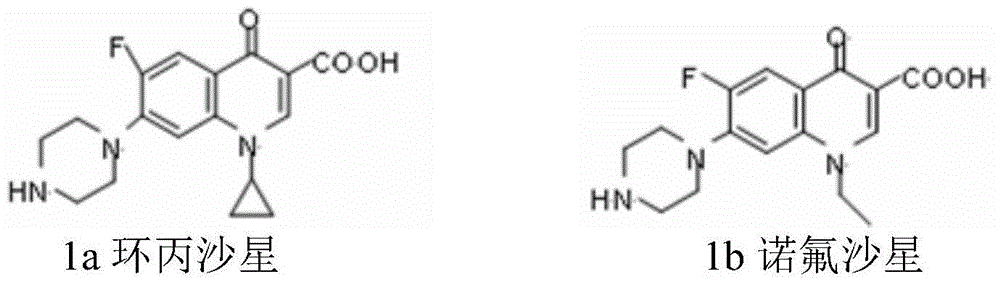
[0033] Example 1: Preparation of ciprofloxacin methyl ester (compound 2a)
[0034] In the flask, add 1.35g (4mmol) of ciprofloxacin, 50mL of methanol, slowly add 2mL of concentrated sulfuric acid dropwise, reflux for 24h, evaporate most of the solvent, adjust the pH to 9 with saturated sodium bicarbonate solution, and then use CH 2 Cl 2 (3×20mL) Extract, combine the organic layers, wash with water to pH 8, and use anhydrous Na for the organic layer 2 SO 4 Dry for several hours, filter, evaporate the solvent under reduced pressure and use silica gel column chromatography (eluent is V (Methanol) : V (Dichloromethane) = 1:10) Purified to obtain 0.704 g of methyl ciprofloxacin (white solid), with a yield of 51%, and a melting point of 253-254°C.
[0035] With reference to the method of Example 1 above, other methyl esters of floxacin (norfloxacin methyl ester, enoxacin methyl ester, clinfloxacin methyl ester) were prepared as follows:
[0036] Repeat Example 1, the difference is: ① repl...
Embodiment 2
[0039] Example 2: Preparation of ferrocene carbonyl chloride (compound 3)
[0040] Add 4.6g (0.02mmol) of dry ferrocene formic acid and 80mL of anhydrous benzene into a three-necked flask. Under the protection of nitrogen, slowly add 6.24g (0.03mmol) of phosphorus pentachloride in several times, stir at room temperature for 3h, and reduce pressure. The benzene was distilled off, and petroleum ether (60-90°C) was extracted to obtain a dark red solution, which was distilled under reduced pressure and cooled to precipitate dark red needle-like crystals. The yield was 80%, mp: 48-49°C.
Embodiment 3
[0041] Example 3: (7-(4-ferrocenecarbonyl-piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid methyl ester (chemical name: Preparation of 7-(4-ferrocenyl piperazinyl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-quinolone-3-carboxylic acid methyl ester) (Compound 4a)
[0042]
[0043] Add 0.345 g (1 mmol) of ciprofloxacin methyl ester, 15 mL of anhydrous THF, 0.4 mL of triethylamine, and nitrogen protection in a dry double-necked flask. Stir under an ice water bath. Use a syringe to slowly inject 5 mL of ferrocene formyl chloride ( 1.2mmol) in anhydrous THF solution, after 10 minutes, transfer to room temperature and stir for 24h, and then filter with suction to obtain compound 4a (if not pure, use silica gel column chromatography (eluent is V (Methanol) : V (Dichloromethane) = 1:20) Purification). Yellow solid, mp: 186~189℃, yield 72%. IR(KBr)v / cm -1 : 3450.20,1725.72,1619.44,1480.06,1443.05,1384.21,1332.78,1258.77,1169.55,1105.32,1011.04,932.58,80...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com